the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
A novel specimen-based mid-Paleozoic dataset of antiarch placoderms (the most basal jawed vertebrates)
Zhaohui Pan
Zhibin Niu
Zumin Xian
Antiarcha data are essential to quantitative studies of basal jawed vertebrates. The absence of structured data on key groups of early vertebrates, such as Antiarcha, has lagged in understanding their diversity and distribution patterns. Previous works of early vertebrates usually focused on anatomy and phylogeny, given their significant impacts on the evolution of key characters, but lacked comprehensive structured data. Here, we contribute an unprecedented open-access Antiarcha dataset covering 60 genera of 6025 specimens from the Ludfordian to the Famennian globally. We have organized an expert team to collect and curate 142 publications spanning from 1939 to 2021. Additionally, we have two-stage quality controls in the process: domain experts examined the literature and senior experts reviewed the results. In this paper, we give details of the data storage structure and visualize these antiarch fossil sites on the paleogeographic map. The novel Antiarcha dataset has tremendous research potential, including testing previous qualitative hypotheses in biodiversity changes, spatiotemporal distribution, evolution, and community composition. It is now an essential part of the DeepBone database and will be updated with the latest publication, also available on https://doi.org/10.5281/zenodo.6536446 (Pan and Zhu, 2021).
- Article
(6610 KB) - Full-text XML
-
Supplement
(337 KB) - BibTeX
- EndNote
Placodermi is an extinct group of jawed vertebrates that first occurred in the Silurian, then dominated the Devonian and constituted a prevalent biotic component of the marine vertebrate ecosystem from 425.0 to 358.9 million years ago (Carr, 1995; Denison, 1978; Janvier, 1996; Young, 2010; Zhu, 1996). Recent prevailing phylogenetic hypotheses placed Placodermi as jawed stem-Gnathostomata that is sister to crown-Gnathostomata or modern jawed vertebrates (Brazeau, 2009; Davis et al., 2012; Dupret et al., 2014; Giles et al., 2015; King, 2021; Long et al., 2015; Qiao et al., 2016; Trinajstic et al., 2015; Zhu et al., 2013, 2016). In this scenario, Antiarcha has usually been placed at the most basal position in the Placodermi (Fig. 1), representing the most basal jawed vertebrates. The spatiotemporal distribution of Antiarcha will thus help us understand the origin and early evolution of jawed vertebrates. For example, Sallan et al. (2018) found that vertebrate diversification occurred primarily in nearshore environments, by analyzing early vertebrate occurrence and habitat data. Historically, antiarchs resided in various paleoenvironments across all paleocontinents, including marine and freshwater environments close to shore. As a successful vertebrate group during the Devonian (Long, 2011; Young, 2010), Antiarcha has contributed significantly to the Devonian stratigraphic correlation. For instance, the biozonation of the East Baltic and southern East Antarctica Devonian succession is partly based upon the antiarchs Bothriolepis, Asterolepis, and Pambulaspis (Young, 1974, 1988). Lukševičs (1996) identified 14 bothriolepid species (12 Bothriolepis and two Grossilepis) in the Frasnian–Famennian formations of the East European Platform, proposed nine antiarch assemblages, and set up the most detailed zonation of the Main Devonian Field, northwestern part of the East European Platform (Latvia and NW Russia).
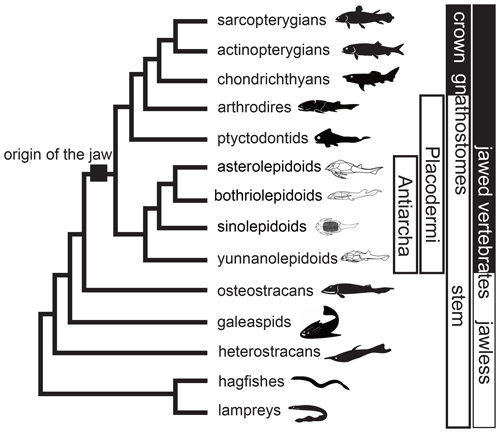
Figure 1Phylogenetic relationships of major early vertebrate groups from Qiao et al. (2016) and Pan et al. (2018). Silhouettes indicate groups of Antiarcha.
Collecting and visualizing the data of Antiarcha is a prerequisite to explaining the spatial and temporal distribution of early vertebrates. With the help of data visualization, we could better understand the biogeographic evolution of early vertebrates. Although Zigaite and Blieck (2013) advocated a quantitative analysis to define early vertebrates' biogeographic patterns, efficient quantitative analysis is still lacking to understand the dispersal of early vertebrates. This occurs mainly because no comprehensive data collection of early vertebrates was accomplished. What is more, the disadvantages of unstructured data are clear: the absence of schema and structure makes them difficult to manage, and the lack of predefined attributes makes them difficult to be reused or extended.
In this paper, we present an unprecedented structured dataset of Antiarcha that potentially facilitates understanding the spatiotemporal distribution pattern and quantifying the variety of antiarchs. This dataset is open-access and follows the FAIR principles (findability, accessibility, interoperability, and reusability) (Wilkinson et al., 2016). This dataset complements existing fossil records of early vertebrates. Moreover, it is the first step to accomplishing the global coverage of the vertebrate fossil dataset to analyze the Middle Paleozoic biogeography and paleogeography. Visualizing the distribution of antiarchs in the paleogeographic background with reference to the global paleogeographic reconstructions of Scotese (2021), our preliminary results can also be used to test the hypothesis of paleogeographic reconstructions.
2.1 Overview
Comprehensive data are essential for quantitative studies and simulation analysis on early vertebrates. Sallan et al. (2018) pointed out that a lack of early vertebrate fossil data has limited quantitative approaches and hindered the resolution of issues regarding ancestral habitat in vertebrate evolution. To bring the study of vertebrate paleontology into the next phase of macroevolution, we built the DeepBone database with the implementation of a project entitled “Big Earth Data Science Engineering (CASEarth)” in 2018 (Guo, 2017; Pan and Zhu, 2019).
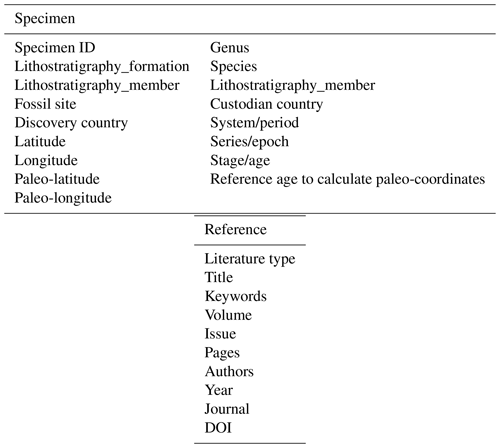
With continuously refining data, the Antiarcha dataset of the DeepBone database is the first and most comprehensive dataset endorsed by Chinese researchers at the Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences. The Antiarcha dataset of DeepBone differs from that of PBDB (https://paleobiodb.org/, last access: 22 October 2021) in its basic unit, which is the specimen ID coupled with the occurrence and other detailed data. All the specimens are referenced in taxa and literature to guarantee accuracy. Because the data format was designed as specimen-based, we input the metadata according to the published specimen ID or virtual specimen ID. The literature on classic systematic paleontology always has real specimen IDs. When it handled stratigraphic topics, the authors usually cited fossil records instead of real specimens. We introduce a virtual specimen ID to store the taxon information in this kind of literature containing no real specimens.
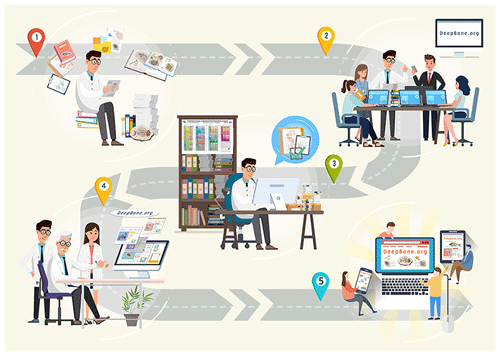
Figure 2Workflow of the data processing. (1) Collecting and sifting lectures by experienced experts. (2) Data entry assistants digitalize paleontological descriptions from the page into the DeepBone database. (3) According to the reference, experts are accountable for data review and cleaning to implement quality control. (4) Senior researchers review the data again. (5) Data managers release the data to the public through DeepBone.org (http://DeepBone.org, last access: 22 October 2021).
Since no satisfactory approach can automatically extract paleontological data from the literature, we recruit several data entry assistants, including researchers, master's students, and PhD students to collect and curate the data. In order to guarantee the quality of the data, we designed a four-step data processing procedure (Fig. 2) as follows.
-
Experts who obtained their PhD degrees in paleontology collected and sifted the data source.
-
Data entry assistants read the related references, extracted the antiarch placoderm data, and manually filled them into the online record file under the supervision of vertebrate paleontology experts.
-
According to the references, experts reviewed and cleaned the data line by line as the quality control procedure.
-
Senior experts, who have outstanding achievements in vertebrate paleontology, reviewed the data again to guarantee quality.
-
DeepBone.org (http://DeepBone.org, last access: 22 October 2021) published the dataset with visualization. A better user interface helps dissemination.
Next, we provide more details on the data processing and visualization.
2.2 Data source
The data were extracted from the published literature containing information on antiarch specimens. Most of the journals are professional journals on paleontology. The main journals include Alcheringa, Acta Geologica Polonica, Bulletin of the Geological Society of China, Estonian Journal of Earth Sciences, Journal of Vertebrate Paleontology, Journal of Paleontology, Palaeontologia Electronica, Palaeontology, Palaeoworld, and Vertebrata PalAsiatica. In total, we have collected 142 publications spanning from 1939 to 2021 (see dataset for more details). The satisfactory literature should include an accurate description or revision of the specimen and taxon. We accepted the latest peer-reviewed literature to deal with the inconsistent descriptions of stratigraphy and taxonomy.
2.3 Data processing and quality control
We made a tailored web page that provides a better user interface for data entry assistants to fill in the rows of paleontological data. After that, the other related experts would review the data so that a researcher could quickly access them to perform quantitative analysis (Fig. 2). This workflow was adopted from the Geobiodiversity Database (GBDB) (Xu et al., 2020). Almost all antiarch literature was published in English, Russian, French, German, and Chinese. The data entry assistant could handle the literature in Chinese and English well. Many fossils were documented in French, Russian, and German. We invited paleontology postgraduates who know French, Russian, or German to deal with the literature in these languages.
The faunistic elements in the communities are used herein at the genus level for their distributions because many Bothriolepis and Asterolepis species were described based on isolated plates lacking diagnostic characters (Blieck and Janvier, 1993; Downs, 2011). Identifying a specimen depends on the ability to recognize species in a way that is coherent within a particular genus and through broader groups. This is very difficult for fossil material because of two especially intractable problems: practically because of the fragmentary nature of the fossil and philosophically because of questions with the criteria by which fossil species (Nelson, 1984; Thomson and Thomas, 2001) are demarcated. For example, Thomson and Thomas (2001) reviewed the previous study on Bothriolepis and proposed that B. nitida, B. minor, B. virginiensis, B. darbiensis, and B. coloradensis could not be consistently distinguished. Weems (2004) questioned the validity of B. virginiensis. Since there is no consensus on the species level of Bothriolepis and Asterolepis, the former researchers only used the evidence of Antiarcha on the genus level to discuss the biostratigraphic significance (Lelievre and Goujet, 1986; Pan, 1981; Young et al., 2010; Young and Lu, 2020). To keep accuracy and consistency, here we choose the genus of Antiarcha to perform data visualization.
2.4 Data visualization
Due to the easy access of the paleogeographic coordinates calculator (PointTrack version 7.0) (Scotese, 2021) and its wide use in paleontology (Ke et al., 2016; Kiel, 2017), we decided to use Scotese's paleocontinent reconstruction to perform the plot map, although many paleogeographic reconstructions were proposed (Heckel and Witzke, 1979; Li and Powell, 2001). Using the TrackPoint software, we converted the excavation locations from the current GPS to paleo-GPS and visualized the locations using the Web Mercator algorithm (Battersby et al., 2014).
The timescale follows the International Commission on Stratigraphy International Chronostratigraphic Chart version 2021/07 (Cohen et al., 2013).
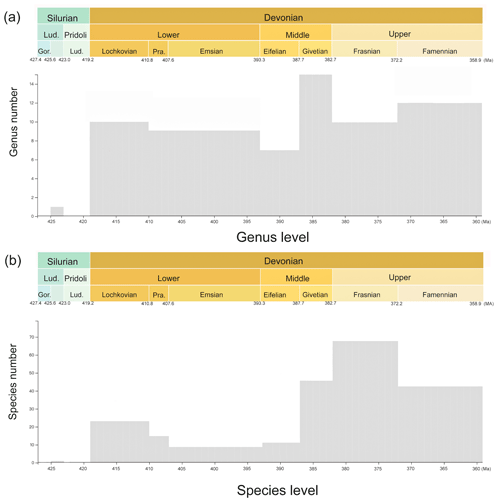
Figure 3Histogram of specimen number. (a) The genus number at different time intervals and (b) the species number at different time intervals.
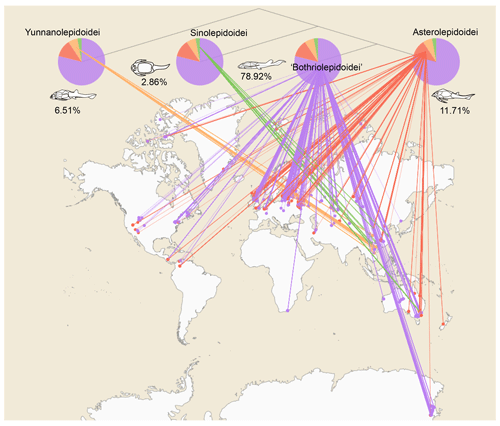
Figure 4Antiarch placoderm spatial distribution. The tree topology is simplified from the phylogenetic result in Pan et al. (2018). Numbers under the pie chart represent each group's relative amounts of specimens. Terminal groups are linked with their geographic distributions. Each node represents a single specimen. Specimens from one locality overlap each other.
3.1 Data overview
This dataset consists of 60 genera of 6025 specimens, covering all known antiarch lineages. The observed quantities of genus and species in our dataset over time were summarized in histograms (Fig. 3). The 6025 specimens include 5867 fossil specimens that have been systematically described and documented and 158 virtual specimens introduced to describe the taxon information when no specimen was assigned for the referred fossil records. Each specimen has at least one reference within our dataset, and the specimens lacking precise age are excluded. We followed the lithostratigraphic information of the original authors except we found a revision. We accepted the latest revision in the literature to modify our dataset. The amendments were linked to the latest reference as an endorsement. We took the geological background data in our dataset unless they were missing from the original literature. We transferred the unstructured data from the literature to structured data for further research in as much detail as possible. Table 1 shows the data structure of our present dataset. Among all the referred specimens, 6.51 % belong to Yunnanolepidoidei, 2.86 % belong to Sinolepidoidei, 78.92 % belong to “Bothriolepidoidei”, and 11.71 % belong to Asterolepidoidei. We plotted all the fossil sites of the constituent groups in Fig. 4.
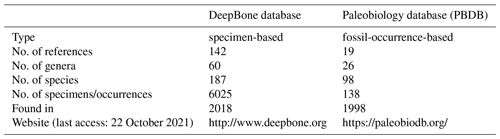
GBDB is a stratigraphic and paleontological database, but no antiarch record exists. Compared to the 138 records of Antiarcha in the Paleobiology Database (PBDB, 22 October 2021), this is the most comprehensive dataset of Antiarcha up to now (Table 2). Only taxon rank, reference, and occurrence location are available in PBDB. DeepBone dataset has more fields on the structured information of the specimen than in the PBDB, such as lithostratigraphic fields (Table 1). Some records in PBDB are not stored at the genus or species level. There are some typing errors in PBDB, for instance, “Jiangxilepus”, “Bothriolepiodei”, and “Pterichthys”. Jiangxilepis, “Bothriolepidoidei”, and Pterichthyodes are the correct spellings. Macrodontophion is not a genus of Antiarcha, but PBDB adopts it in antiarchs. PBDB also adopts Silurolepis as an antiarch, ignoring the latest research of Zhu et al. (2019). To ensure accuracy, every specimen of DeepBone is endorsed by the latest publication and reviewed by the experts who have focused on Antiarcha. It is open to access through the website of the DeepBone database or https://doi.org/10.5281/zenodo.6478602 (Pan and Zhu, 2021).
3.2 The geospatial distribution of the Antiarcha dataset
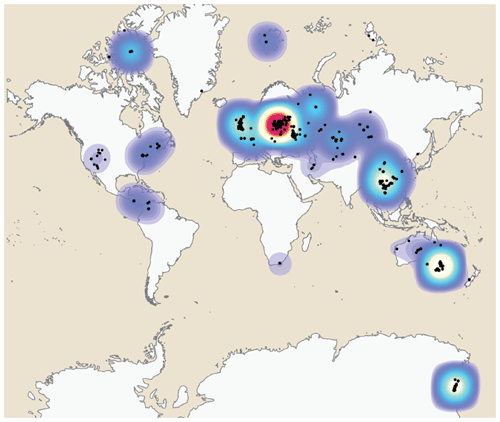
The geospatial distribution of the Antiarcha is shown in Fig. 4. Yunnanolepidoidei is endemic in the South China block (comprising southern China and northern Vietnam) regarding the fossil site distribution. Sinolepidoidei is limited in South China and Australia (East Gondwana). In contrast, “Bothriolepidoidei” and Asterolepidoidei are cosmopolitan, especially Bothriolepis. The heat map of fossil sites (Fig. 5) shows that Europe, Australia, and China account for the most fossil sites globally, partly due to their long research history.
3.3 The paleogeographic distribution of the Antiarcha dataset
As Young (1990) mentioned that biogeographic data must be interpreted in the context of paleogeographic hypotheses, we plot our data on a paleogeographic atlas (Fig. 6) only to generate an outline of their past. The further interpreting data studies will be published separately. The continental reconstructions of Scotese place Baltica, China, and Australia in the tropics and subtropics near the paleo-Equator from Llandovery to Famennian. We excluded the Silurian Shimenolepis because it is the earliest record of Yunnanolepidoidei and the only documented antiarch specimen before the Devonian (Wang, 1991; Zhao et al., 2016). Most of the fossil sites were positioned around the paleo-Equator. In the present scenario, the suborder Yunnanolepidoidei apparently originated as early as the Silurian in the South China block, forming a highly endemic fauna. All fossil sites of Yunnanolepidoidei lay in southern China and northern Vietnam (Wang et al., 2010). From Ludlow (Silurian) to the Early Devonian, Yunnanolepidoidei formed dominant antiarchs. Sinolepidoidei and “Bothriolepidoidei” first appeared in Pragian in South China, and Asterolepidoidei first evolved in Emsian in Australia or East Gondwana. During the middle Devonian, along with lessened isolation of South China, Yunnanolepidoidei became extinct. Euantiarcha (“Bothriolepidoidei” + Asterolepidoidei) dominated Middle and Late Devonian antiarchs, and only a few members of Sinolepidoidei coexisted with them in China and Australia. In Eifelian, Asterolepidoidei suddenly bloomed in Baltica without any clue from the older horizons based on existing research. The distribution of Antiarcha reached a peak in Givetian. “Bothriolepidoidei”, and Asterolepidoidei represent the main groups of Antiarcha in Givetian, comprising five bothriolepidoid genera with 42 fossil locations and nine asterolepidoid genera with 49 fossil locations.
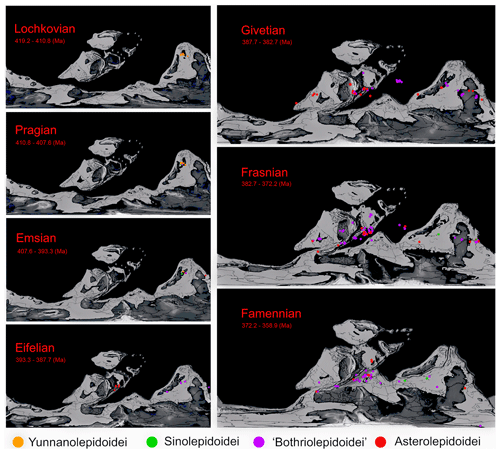
Figure 6The distributions of Antiarcha during Devonian. Each spot represents the location of one specimen on the paleogeographic map. Specimens from the same locality and the same age overlap each other. The paleo-coordinates are calculated by TrackPoint. Colors denoting respective groups follow Fig. 4.
3.4 First appearance record
A taxon's first appearance record or lineage is important in paleontology and evolutionary biology. It renders a hard minimum constraint on molecular clock calibration for a taxon (Benton and Donoghue, 2007; Benton et al., 2009; Donoghue and Benton, 2007). Based on our dataset, the oldest record of yunnanolepidoids or antiarchs is Shimenolepis graniferous from the Xiaoxi Formation at Shanmen Reservoir, Lixian County, Hunan, China. Shimenolepis was first described as the oldest known placoderm, dated as Telychian of Llandovery (Janvier, 1996; Wang, 1991). However, after a detailed stratigraphic work, Zhao et al. (2016) suggested that the age of Shimenolepis is late Ludlow rather than late Llandovery. Janvier and Tông-Dzuy (1998) also documented an indeterminate yunnanolepidoid (Antiarcha gen. sp. indet.) from the Do Son Formation of northern Vietnam, which could be another earliest antiarch potentially.
The oldest sinolepid is Liujiangolepis suni, from the Nakaoling Formation (Pragian), Xiangzhou, Guangxi, China (Wang, 1987). The oldest bothriolepidid is Houershanaspis zhangi, documented from the Danlin Formation (Pragian) in Houershan, Dushan county, Guizhou, southwestern China, based on a bothriolepid-like anterior median dorsal plate (Lu et al., 2017). The earliest asterolepidoid records are represented by Wurungulepis and some disarticulated specimens, which have been documented from the Broken River Formation, Broken River, Australia. The age of the Broken River Formation was first referred to Eifelian and then reassigned to Emsian (serotinus Zone) (Burrow, 1996; De Pomeroy, 1996; Young, 1984, 1990).
The current dataset achieved via Zenodo represents a static version of the dataset in October 2022 (https://doi.org/10.5281/zenodo.6536446 (Pan and Zhu, 2021). The latest version of the dataset is always freely available via https://deepbone.org/ (last access: 22 October 2022).
We presented here an open-access dataset of Antiarcha, the most basal jawed vertebrate, from the late Silurian to the Late Devonian. This dataset significantly expands the previously available data of antiarch fossils. Paleontologists, stratigraphers, and evolutionary biologists could import the tab-delimited file for future research studies, especially for biodiversity analysis, stratigraphic correlation, and molecular clock calibration. With the information of 6025 specimens, our Antiacha dataset is far more comprehensive than the other sources in lithostratigraphy and specimen details. Data are significant for quantitative analysis and potentially contribute to data-driven paleontology research. We performed a visualization of the data to show the spatiotemporal distribution of Antiarcha. In brief, Antiarcha first appeared in the Pan-Cathaysia province during the late Ludlow and then boomed worldwide. At the end of Devonian, Antiarcha was extinct along with the traditional placoderms. The available Antiarcha data may be just the tip of the historical reality due to the incomplete fossil record. We will continue to update the dataset with the latest publication.
The supplement related to this article is available online at: https://doi.org/10.5194/essd-15-41-2023-supplement.
MZ supervised the project. ZP and ZN performed the visualization. ZP prepared and revised the paper with contributions from MZ and ZN. ZP and ZX prepared the data.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We thank Wenjin Zhao, Zhikun Gai, and Tuo Qiao, Institute of Vertebrate Paleontology and Paleoanthropology (CAS); Junxuan Fan, School of Earth Sciences and Engineering, Nanjing University; Honghe Xu, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (CAS); and Junqi Wu and Yaqi Xue, College of Intelligence and Computing, Tianjin University, for discussions and help.
This project was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant nos. XDA19050102 and XDB26000000), the National Science Foundation of China (grant no. 42002015), the Youth Innovation Promotion Association CAS (grant no. 2021070), and the State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (grant no. 193121).
This paper was edited by Kirsten Elger and reviewed by Mohamad Bazzi and one anonymous referee.
Battersby, S. E., Finn, M. P., Usery, E. L., and Yamamoto, K. H.: Implications of web Mercator and its use in online mapping, Cartographica: J. Geogr. Inf. Geovis., 49, 85–101, https://doi.org/10.3138/carto.49.2.2313, 2014.
Benton, M. J. and Donoghue, P. C. J.: Paleontological evidence to date the tree of life, Mol. Biol. Evol., 24, 26–53, https://doi.org/10.1093/molbev/msl150, 2007.
Benton, M. J., Donoghue, P. C. J., and Asher, R. J.: Calibrating and constraining molecular clocks, in: The Timetree of Life, edited by: Hedges, S. B. and Kumar, S., Oxford Univ. Press, Oxford, 35–86, 2009.
Blieck, A. and Janvier, P.: Silurian-Devonian vertebrate biostratigraphy of Siberia and neighbouring areas, in: Palaeozoic Vertebrate Biostratigraphy and Biogeography, edited by: Long J. A., Bellhaven Press, London, 87–103, 1993.
Brazeau, M. D.: The braincase and jaws of a Devonian “acanthodian” and modern gnathostome origins, Nature, 457, 305–308, https://doi.org/10.1038/nature07436, 2009.
Burrow, C. J.: Placoderm scales from the Lower Devonian of New South Wales, Australia, Mod. Geol., 20, 351–369, 1996.
Carr, R. K.: Placoderm diversity and evolution, Bull. Mus. Natl. Hist. Nat., Sect. C, 17, 85–125, 1995.
Cohen, K. M., Finney, S. C., Gibbard, P. L., and Fan, J. X.: The ICS International Chronostratigraphic Chart, Episodes, 36, 199–204, 2013.
Davis, S. P., Finarelli, J. A., and Coates, M. I.: Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes, Nature, 486, 247–250, https://doi.org/10.1038/nature11080, 2012.
Denison, R. (Ed.): Placodermi, Handbook of Paleoichthyology, vol. 2, Verlag Dr. Friedrich Pfeil, New York, ISBN 9783899370379, 1978.
De Pomeroy, A. M.: Biostratigraphy of Devonian microvertebrates from the Broken River region, north Queensland, West. Aust. Mus. Rec., 17, 417–437, 1996.
Donoghue, P. C. J. and Benton, M.: Rocks and clocks: calibrating the tree of life using fossils and molecules, Trends Ecol. Evol., 22, 424–431, https://doi.org/10.1016/j.tree.2007.05.005, 2007.
Downs, J. P.: Mass mortality of juvenile antiarchs (Bothriolepis sp.) from the Catskill Formation (Upper Devonian, Famennian Stage), Tioga County, Pennsylvania, P. Acad. Nat. Sci. Phila., 161, 191–203, 2011.
Dupret, V., Sanchez, S., Goujet, D., Tafforeau, P., and Ahlberg, P. E.: A primitive placoderm sheds light on the origin of the jawed vertebrate face, Nature, 507, 500–503, https://doi.org/10.1038/nature12980, 2014.
Giles, S., Friedman, M., and Brazeau, M. D.: Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome, Nature, 520, 82–85, https://doi.org/10.1038/nature14065, 2015.
Guo, H. D.: Big Earth data: A new frontier in Earth and information sciences, Big Earth Data, 1, 4–20, https://doi.org/10.1080/20964471.2017.1403062, 2017.
Heckel, P. H. and Witzke, B. J.: Devonian world palaeogeography determined from distribution of carbonates and related lithic palaeoclimatic indicators, in: The Devonian System, edited by: House M. R., Vol. Spec. Pap. Paleontol., 23, 99–123, 1979.
Janvier, P. (Ed.): Early Vertebrates, Clarendon Press, Oxford, United States, ISBN-10 0198526466, 1996.
Janvier, P. and Tông-Dzuy, T.: The Silurian and Devonian vertebrates of Viêt Nam: a review, J. Geol. B, 1998, 18–28, 1998.
Ke, Y., Shen, S. Z.., Shi, G. R., Fan, J. X., Zhang, H., Qiao, L., and Zeng, Y.: Global brachiopod palaeobiogeographical evolution from Changhsingian (Late Permian) to Rhaetian (Late Triassic), Paleogeogr. Paleocl., 448, 4–25, https://doi.org/10.1016/j.palaeo.2015.09.049, 2016.
Kiel, S.: Using network analysis to trace the evolution of biogeography through geologic time: A case study, Geology, 45, 711–714, 2017.
King, B.: Bayesian tip-dated phylogenetics in paleontology: topological effects and stratigraphic fit, Syst. Biol., 70, 283–294, https://doi.org/10.1093/sysbio/syaa057, 2021.
Lelièvre, H. and Goujet, D.: Biostratigraphic significance of some uppermost Devonian Placoderms, Annales de la Société de Géologie de Belgique, 109, 55–59, 1986.
Li, Z. X. and Powell, C. M.: An outline of the palaeogeographic evolution of the Australasian region since the beginning of the Neoproterozoic, Earth-Sci. Rev., 53, 237–277, https://doi.org/10.1016/S0012-8252(00)00021-0, 2001.
Long, J. A.: Dawn of the Deed. Sci. Am., 304, 34–39, 2011.
Long, J. A., Mark-Kurik, E., Johanson, Z., Lee, M. S., Young, G. C., Zhu, M., Ahlberg, P. E., Newman, M., Jones, R., Blaauwen, J., Choo, B., and Trinajstic, K.: Copulation in antiarch placoderms and the origin of gnathostome internal fertilization, Nature, 517, 196–199, https://doi.org/10.1038/nature13825, 2015.
Lu, L., Tan, K., and Wang, X.: A New Antiarchi (placoderm fishes) from Devonian Strata of Dushan, Guizhou Province, Acta Geosci. Sin., 38, 144–148, 2017.
Lukševičs, E.: Bothriolepid antiarchs (Placodermi, Bothriolepididae) from the north-western part of East European platform, PhD thesis, Geogrāfijas un Zemes zinātņu fakultāte, University of Latvia, Latvia, 196 pp., 1996.
Nelson, J. S.: Fishes of the World, 2nd edn., Jonh Wiley & Sons, New York, 523 pp., ISBN 0-471-86475-7, 1984.
Pan, K.: Devonian antiarch biostratigraphy of China, Geol. Mag., 118, 69–75, 1981.
Pan, Z. and Zhu, M.: Dataset of antiarch placoderms (the most basal jawed vertebrates) throughout Middle Paleozoic, Zenodo [data set], https://doi.org/10.5281/zenodo.6536446, 2021.
Pan, Z. H. and Zhu, M.: VPPDB: Hosting the data for vertebrate paleoanthropology, Acta Geol. Sin.-Engl., 93, 61–63, https://doi.org/10.1111/1755-6724.14246, 2019.
Pan, Z. H., Zhu, M., Zhu, Y. A., and Jia, L. T.: A new antiarch placoderm from the Emsian (Early Devonian) of Wuding, Yunnan, China, Alcheringa, 42, 10–21, https://doi.org/10.1080/03115518.2017.1338357, 2018.
Qiao, T., King, B., Long, J. A., Ahlberg, P. E., and Zhu, M.: Early gnathostome phylogeny revisited: multiple method consensus, PLoS One, 11, e0163157, https://doi.org/10.1371/journal.pone.0163157, 2016.
Sallan, L., Friedman, M., Sansom, S. R., Bird, M. C., and Sansom, J. I.: The nearshore cradle of early vertebrate diversification, Science, 362, 460–464, https://doi.org/10.1126/science.aar3689, 2018.
Scotese, C. R.: Tutorial: PALEOMAP paleoAtlas for GPlates and the paleoData plotter program, Technical Report, 56, https://www.earthbyte.org/paleomap, last access: 31 August 2021.
Thomson, K. S. and Thomas, B.: On the status of species of Bothriolepis (Placodermi, Antiarchi) in North America, J. Vertebr. Paleontol., 21, 679–686, https://doi.org/10.1671/0272-4634(2001)021[0679:OTSOSO]2.0.CO;2, 2001.
Trinajstic, K., Boisvert, C., Long, J., Maksimenko, A., and Johanson, Z.: Pelvic and reproductive structures in placoderms (stem gnathostomes), Biol. Rev., 90, 467–501, https://doi.org/10.1111/brv.12118, 2015.
Wang, J. Q.: The Antiarchi from Early Silurian of Hunan, Vertebr. Palasiat., 29, 240–244, 1991.
Wang, S. T.: A new antiarch from the Early Devonian of Guangxi, Vertebr. Palasiat., 25, 81–90, 1987.
Wang, W., Qu, Q. M., and Zhu, M.: A brief review of the Middle Palaeozoic vertebrates from Southeast Asia, Palaeoworld, 19, 27–36, https://doi.org/10.1016/j.palwor.2009.12.005, 2010.
Weems, R. E.: Bothriolepis virginiensis, a valid species of placoderm fish separable from Bothriolepis nitida, J. Vertebr. Paleontol., 24, 245–250, 2004.
Wilkinson, M. D., Dumontier, M., Aalbersberg, I. J., Appleton, G., Axton, M., Baak, A., Blomberg, B., Boiten, J., Santos, L. B. S., Bourne, P. E., Bouwman, J., Brookes, A. J., Clark, T., Crosas, M., Dillo, I., Dumon, O., Edmunds, S., Evelo, C. T., Finkers, R., Gonzalez-Beltran, A., Gray, A. J. G., Growth, P., Goble, C., Grethe, J. S., Heringa, J., Hoen, P. A. C., Hooft, R., Kuhn, T., Kok, R., Kok, J., Lusher, S. J., Martone, M. E., Mons, A., Packer, A. L., Persson, B., Rocca-Serra, P., Roos, M., Schaik, R. V., Sansone, S. A., Schultes, E., Sengstag, T., Slater, T., Strawn, G., Swertz, M. A., Thompson, M., Lei, J. V. D., Mulligen, E. V., Velterop, J., Waagmeester, A., Wittenburg, P., Wolstencroft, K., Zhao, J., and Mons, B. : The FAIR Guiding Principles for scientific data management and stewardship, Sci. Data, 3, 1–9, 2016.
Xu, H.-H., Niu, Z.-B., and Chen, Y.-S.: A status report on a section-based stratigraphic and palaeontological database – the Geobiodiversity Database, Earth Syst. Sci. Data, 12, 3443–3452, https://doi.org/10.5194/essd-12-3443-2020, 2020.
Young, G. C.: Stratigraphic occurrence of some placoderm fishes in the Middle and Late Devonian, Newsl. Stratigr., 3, 243–261, 1974.
Young, G. C.: An asterolepidoid antiarch (placoderm fish) from the Early Devonian of the Georgina Basin, central Australia, Alcheringa, 8, 65–80, 1984.
Young, G. C.: Antiarchs (placoderm fishes) from the Devonian Aztec siltstone, southern Victoria Land, Antarctica, Palaeontogr. Abt. A, 202, 1–125, 1988.
Young, G. C.: Devonian vertebrate distribution patterns and cladistic analysis of palaegeographic hypotheses, Geol. Soc. Lond., Mem., 12, 243–255, https://doi.org/10.1144/gsl.mem.1990.012.01.23, 1990.
Young, G. C.: Placoderms (armored fish): dominant vertebrates of the Devonian period, Annu. Rev. Earth Pl. Sc., 38, 523–550, https://doi.org/10.1146/annurev-earth-040809-152507, 2010.
Young, G. C. and Lu, J.: Asia–Gondwana connections indicated by Devonian fishes from Australia: palaeogeographic considerations, J. Vertebr. Paleontol., 9, 1–22, 2020.
Young, G. C., Burrow, C. J., Long, J. A., Turner, S., and Choo, B.: Devonian macrovertebrate assemblages and biogeography of East Gondwana (Australasia, Antarctica), Palaeoworld, 19, 55–74, https://doi.org/10.1016/j.palwor.2009.11.005, 2010.
Zhao, W. J., Zhu, M., Liu, S., Pan, Z. H., and Jia, L. T.: A new look at the Silurian fish-bearing strata around the Shanmen Reservoir in Lixian, Hunan province, J. Stratigr., 40, 341–350, 2016.
Zhu, M.: The phylogeny of the Antiarcha (Placodermi, Pisces), with the description of Early Devonian antiarchs from Qujing, Yunnan, China, Bull. Mus. Natl. Hist. Nat., Sect. C, 18, 233–347, 1996.
Zhu, M., Yu, X. B., Ahlberg, P. E., Choo, B., Lu, J., Qiao, T., Qu, Q. M., Zhao, W. J., Jia, L. T., Blom, H., and Zhu, Y. A.: A Silurian placoderm with osteichthyan-like marginal jaw bones, Nature, 502, 188–193, https://doi.org/10.1038/nature12617, 2013.
Zhu, M., Ahlberg, P. E., Pan, Z. H., Zhu, Y. A., Qiao, T., Zhao, W. J., Jia, L. T., and Lu, J.: A Silurian maxillate placoderm illuminates jaw evolution, Science, 354, 334–336, https://doi.org/10.1126/science.aah3764, 2016.
Zhu, Y. A., Lu, J., and Zhu, M.: Reappraisal of the Silurian placoderm Silurolepis and insights into the dermal neck joint evolution, R. Soc. Open Sci., 6, 191181, https://doi.org/10.1098/rsos.191181, 2019.
Zigaite, Z. and Blieck, A.: Chapter 28 Palaeobiogeography of Early Palaeozoic vertebrates, Geol. Soc. Lond., Mem., 38, 449–460, https://doi.org/10.1144/m38.28, 2013.