the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Database of global glendonite and ikaite records throughout the Phanerozoic
Victoria Ershova
Oleg Vereshchagin
Kseniia Vasileva
Kseniia Mikhailova
Aleksei Krylov
This database of Phanerozoic occurrences and isotopic characteristics of metastable cold-water calcium carbonate hexahydrate (ikaite; CaCO3•6H2O) and their associated carbonate pseudomorphs (glendonites) has been compiled from academic publications, explanatory notes, and reports. Our database including more than 700 occurrences reveals that glendonites characterize cold-water environments, although their distribution is highly irregular in space and time. A significant body of evidence suggests that glendonite occurrences are restricted mainly to cold-water settings; however they do not occur during every glaciation or cooling event of the Phanerozoic. While Quaternary glendonites and ikaites have been described from all major ocean basins, older occurrences have a patchy distribution, which may suggest poor preservation potential of both carbonate concretions and older sediments.
The data file described in this paper is available on Zenodo at https://doi.org/10.5281/zenodo.4386335 (Rogov et al., 2020).
- Article
(7042 KB) - Full-text XML
-
Supplement
(134 KB) - BibTeX
- EndNote
Metastable cold-water calcium carbonate hexahydrate (ikaite; CaCO3•6H2O) and its associated carbonate pseudomorphs (glendonites) have attracted considerable attention during the past few decades, mainly due to their possible utility for paleoenvironmental (especially paleoclimatic) reconstructions (Kemper and Schmitz, 1975, 1981; Kaplan, 1978, 1979, 1980; Suess et al., 1982, among others). Carbonate pseudomorphs of metastable ikaite have acquired a number of different names (anthraconite, glendonite, thinolite, jarrowite, pseudogaylussite, gennoishi, hokou-seki, White Sea hornlets, polar euhedrons, barleycorn, Gerstenkörner, hedgehogs; see Supplement), and despite nearly 200 years of study (since the earliest papers by Sokolov, 1825; Freisleben, 1827; Pander, 1830), their distribution and formation mechanisms are still poorly understood. We use the most common name for these pseudomorphs (glendonite), which is derived from their famous locality, Glendon (NSW, Australia; see Dana, 1849; David et al., 1905). Modern ikaite occurs in a wide range of cold-water environments. Ikaite microcrystals have been recorded in sea ice and cold caves, ikaite tufa has been reported from shallow marine and lacustrine settings, and macrocrystals and their aggregates have been described from hypersaline springs and lacustrine and marine sediments, ranging from littoral environments to abyssal depths (up to ∼ 6950 m) (Pauly, 1963; Stein and Smith, 1986; Ito, 1996; Dieckmann et al., 2008; Last and Last, 2012; Geptner et al., 2014; Fink et al., 2014; Oehlerich et al., 2015). Occurrences of ikaite pseudomorphs (glendonites) in the geological record are characterized by a slightly less diverse range of paleoenvironments. Ancient glendonites have been mainly described from marine clastic rocks, although they have been discovered in caves and lacustrine deposits of Oligocene age (Larsen, 1994).
The first attempts at a comprehensive review of glendonite occurrences through geological time were undertaken by Kaplan (1978, 1980), who summarized the available information about glendonite findings throughout the world and publicized their importance for paleoclimatic studies. For the last 40 years, numerous papers on glendonite and ikaite occurrences have been published, along with new geochemical data. These geochemical data mainly include stable carbon and oxygen isotope values from ikaite and glendonite, along with a limited number of clumped isotope and Sr isotope data (Nenning, 2017; Rogov et al., 2018; Vickers et al., 2020). Information on glendonite occurrences is scattered throughout hundreds of papers on regional geology, explanatory notes to geological maps, and other reports, but until now, no single reference database of global glendonite distribution throughout the Phanerozoic has been compiled.
This paper presents a new single reference database of ikaite and glendonite records from the early Cambrian until the present. It is based on the analysis of numerous papers (including unpublished reports) and museum collections, as well as on data collected over the last 15 years by the authors. All records were averaged to substages (if possible) without further subdivision, and nearby occurrences were considered as a single locality if the distance between separate data points was less than 1–2 km. As glendonite occurrences were frequently mentioned but not imaged or fully described in many publications, consideration of such records as true glendonites was mainly based on additional lines of evidence supporting glendonite occurrence within the same region or stratigraphic interval.
2.1 Mineralogy and petrology
Even though glendonites have been known for more than 200 years (e.g., Sokolov, 1825), the precursor mineral (ikaite) was not discovered until 1963, when Pauly described it from Ikka (Ika) Fjord, Greenland. Single-crystal X-ray structure determinations (e.g., Hesse et al., 1983; Swainson and Hammond, 2001; Lennie et al., 2004) have shown that ikaite crystallizes in the space group , and its structure consists of CaCO3•6H2O units with each Ca bound to six water molecules and a bidentate carbonate group to yield eight-fold coordination. Hydrogen bonding links CaCO3•6H2O moieties to form the crystal structure (Lennie et al., 2004).
Ikaite has been found in natural modern-day environments at temperatures ranging from −2 to +7 ∘C (Dieckmann et al., 2008; Huggett et al., 2005; Suess et al., 1982). The relative abundance of ikaite in nature is attributed to its metastability (e.g., Marland, 1975). Marland (1975) and Shahar et al. (2005) suggest that low temperatures and high pressures are the main factors controlling ikaite stabilization, and they argue that metastable ikaite should not naturally occur on Earth's surface, and other carbonates (e.g., calcite, vaterite, aragonite) should crystallize instead. The main factors proposed for ikaite stabilization at Earth's surface are elevated contents of sulfate, phosphate, and magnesium ions in the crystallization medium, or high pH.
Unfortunately, very few studies have investigated the chemical composition of ikaite (e.g., Suess et al., 1982; Pauly, 1963; Schubert et al., 1997). These few studies did not identify sulfate or phosphate ions as constituents of ikaite, whereas magnesium content ranged up to ∼ 0.5 wt % MgO (Pauly, 1963; Schubert et al., 1997). Schubert et al. (1997) reported minor amounts of Mg, Fe, and Al (540 ± 27, 43.2 ± 1.2, and 19.4 ± 0.2 µg g−1, respectively) in two dried ikaite subsamples, along with a total organic carbon content of 0.15 %. It is important to note that ikaite unit cell parameters provided in published data differ significantly (e.g., Rysgaard et al., 2013), which could indicate chemical composition variations in studied ikaites.
Outside of the aqueous environment, ikaite rapidly disintegrates into a mush of water and anhydrous calcium carbonate (e.g., Pauly, 1963; Bischoff et al., 1993), which can be in the form of amorphous calcium carbonate (e.g., Zou et al., 2018), vaterite (e.g., Shaikh, 1990; Ito, 1996; Ito et al., 1999; Tang et al., 2009), aragonite (e.g., Stein and Smith, 1986; Council and Bennett, 1993), or calcite (e.g., Pauly, 1963; Bischoff et al., 1993). Ito (1998) suggested that the rate of ikaite transformation is dependent on the availability of water (which increases the rate of transformation) and the presence of magnesium ions (which inhibits the transformation to both calcite and vaterite). Tang et al. (2009) argued that transformation of ikaite was controlled structurally and kinetically as the rate of decomposition to vaterite increased with temperature. Purgstaller et al. (2017) showed that the formation of anhydrous calcium carbonates is controlled mainly by the prevailing physicochemical conditions, such as the ratio of the aqueous medium and water availability. Stockmann et al. (2018) confirmed that and showed that the formation of ikaite is unrelated to the aqueous phosphate concentration. On the other hand, several laboratory experiments have shown that phosphate in solution could have an effect on the calcium carbonate crystallization pathway and consequently on ikaite precipitation (Brooks et al., 1950; Bischoff et al., 1993; Hu et al., 2014, 2015). Moreover, an increased content of phosphate ions is often found in pore waters from ikaite-bearing sediment layers (e.g., Kodina et al., 2003).
Ikaite–glendonite transformation begins with ikaite destabilization, resulting in a cloudy (Suess et al., 1982) or whitish (Kodina et al., 2003) interior of the ikaite crystals. The replacement of ikaite by anhydrous calcium carbonate (CaCO3•6H2O → CaCO3+ 6H2O) leads to a primary volume loss of 70 %–80 % (Suess et al., 1982; Fairchild et al., 2016) and disintegration, as the density of ikaite is much less than of anhydrous calcium carbonates. However, the primary morphology of ikaite can be preserved due to fast replacement by calcite and cementation (Huggett et al., 2005; Selleck et al., 2007). Early diagenesis of ikaite typically takes place in the sulfate-reduction zone, and glendonite can be subsequently altered during burial diagenesis, where it can be replaced by younger carbonate generations or non-carbonate minerals such as silica (Wang et al., 2017), dolomite (Loog, 1980), pyrite (Rogala et al., 2007) or gypsum (Mikhailova et al., 2019).
Several mineralogical studies have been performed on glendonites (David et al., 1905; Boggs, 1972; Kaplan, 1979; Larsen, 1994; McLachlan et al., 2001; Greinert and Derkachev, 2004; Huggett et al., 2005; Selleck et al., 2007; Vickers et al., 2020). These studies showed that glendonites are composed of two to four consecutive carbonate phases, which differ in morphology and chemical composition (Fig. 2). Calcite is the only anhydrous calcium carbonate, which was found as a replacive phase after ikaite in natural pseudomorphs (glendonites).
The first carbonate generation comprises blocky calcite crystals, which typically show euhedral, triangular, or bipyramidal shapes, or irregular habits (e.g., Greinert and Derkachev, 2004; Huggett et al., 2005; Qu et al., 2017). Crystals are clear to opaque, with concentric zonation formed by an admixture of clay or organic matter (Huggett et al., 2005), and show no luminescence (e.g., Larsen, 1994; Morales et al., 2017). These crystals are always Mg-, Fe-, and P-depleted (Vickers et al., 2020) or almost pure CaCO3 (Mikhailova et al., 2019). This first generation is interpreted as ikaite-derived calcite (Huggett et al., 2005).

Figure 1Ikaite and glendonite morphology (all specimens are shown at natural size). Abbreviation for museum collections: FMM – Fersman Mineralogical Museum, Moscow; GIN – Geological Institute of RAS, Moscow; NHMO – Natural History Museum, Oslo; TsNIGR – Academician F. N. Chernyshev Central Geological Research Museum, St. Petersburg; SGM – Vernadsky State Geological Museum, Moscow. 1–3 ikaite; 4–12 glendonite. 1 – Laptev Sea, station IP1806, water depth 696 m, level of ikaite sampling 135–137 cm; 2 – Chukchi Sea, station 2-2, water depth 50.4 m, level of ikaite sampling 200–230 cm; 3 – Laptev Sea, station 12, water depth 31 m, level of ikaite sampling 130–140 cm (Krylov et al., 2015); 4 – specimen embedded in concretion attached to glacial boulder, littoral of the White Sea near the Olenitsa River mouth, Holocene (coll. FMM); 5 – glendonite “twins”, Bolshaya Balakhnya (Taimyr), Quaternary (specimen SGM-276-45/MN-61203, coll. by Leopold D. Sulerzhitsky, 1981); 6 – south of Pyatibratsky Cape, western Kamchatka, Eocene (specimen SGM- 445-05/MN-61735, coll. by Aelita I. Chelebaeva, 1988); 7 – Aralskaya River, Sakhalin, upper Eocene (specimen TsNIGR 4/8561, coll. by Irina N. Kuzina); 8 – Tochilinsky section, western Kamchatka, Eocene–Oligocene transition (specimen GIN, coll. by Tatiana N. Palechek); 9 – Basilika mountain, Spitsbergen, lower Albian (specimen NHMO 924, coll. Jenö Nagy, 1964); 10 – Olenek River (near Koluma River mouth), Yakutia, Middle Jurassic, upper Bajocian – lower Bathonian (specimen TsNIGR100/6266, coll. by Aleksei I. Gusev, 1939); 11 – Taas-Ary Island, Yakutia, middle Permian, Roadian (field photo by Victoria B. Ershova); 12 – borehole 60, depth 97.6 m, St. Petersburg region, Lower Ordovician, Tremadocian (specimen TsNIGR 9/13261, coll. by Georgy S. Iskul, 2012).
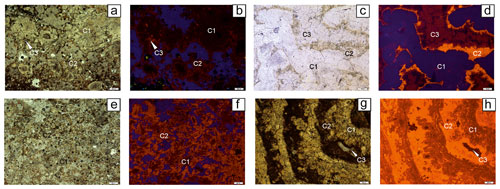
Figure 2The internal structure of glendonites that are mainly composed of several calcite phases (C1; C2; C3). (a–b) Sample L-1-3, Middle Jurassic (Bajocian–Bathonian), Ludlovskaya-1 well, Barents Sea, Russia, depth 1592 + 6 m (authors' data); (a) under plane-polarized light; the first calcite phase represents rosette-like crystals showing distinct core; (b) the same thin section under CL. (c–d) Sample RM2019-27, lower Neogene (lower Miocene), eastern coast of Sakhalin Island, Russia (authors' data); (c) under plane-polarized light; (d) the same thin section under CL. (e–f) Sample Led-1-3, Middle Jurassic (Bajocian–Bathonian), Ledovaya-1 well, Barents Sea, Russia, depth 1824 + 6.6 m (authors' data); (e) elongated calcite crystals (C1), surrounded by the second calcite phase (C2), sample Led-1-3 under plane-polarized light; (f) the same thin section under CL. (g–h) Sample F3-1-1, Lower Cretaceous (upper Hauterivian), Festningen, Svalbard (authors' data); (g) microzoning glendonite under plane-polarized light; the first calcite phase is distinguished zoning as well; (h) the same thin section under CL.
Ikaite-derived calcite does not form the frame of the glendonite and is always supported by cements of different morphology. The first ikaite-derived calcite generation can be overgrown by needle-like or spherulitic calcite cement (e.g., Boggs, 1972; Kaplan, 1979; Huggett et al., 2005; Vickers et al., 2018). Crystals are a yellowish or amber color under the optical microscope with a bright cathodoluminescence (Frank et al., 2008; Teichert and Luppold, 2013, among others). These carbonates are typically Mg- and/or Fe-rich. These carbonates can contain inclusions of pyrite (either as idiomorphic crystals or with framboidal habits; Greinert and Derkachev, 2004). Blocky, sparry, or radiaxial fibrous calcite can occupy the residual pore space (Teichert and Luppold, 2013) or replace carbonate rims and ikaite-derived calcite (Vasileva et al., 2019), typically displaying an orange to dark red cathodoluminescence. The last calcite generation is typically richest in Mg, Fe, Sr, and P (e.g., Vickers et al., 2020)
Besides multiple carbonate generations, some detrital material is also typically found in glendonites. Inclusions of quartz, feldspar (plagioclase/K-feldspar), volcanic glass, olivine, pyroxene, amphibole, magnetite, hematite, and mica can also occur (e.g., Astakhova and Sorochinskaya, 2000). Glendonites also tend to have a high organic matter content, which is enough to be measured for stable isotopes by dissolving the carbonate (e.g., Vickers et al., 2020).
2.2 Morphology
Unlike calcite and aragonite, ikaite is typically characterized by pyramidal, spear-like (e.g., Dieckmann et al., 2008; Tang et al., 2009; Rysgaard et al., 2014), or stellate crystals (e.g., Selleck et al., 2007) with square-prismatic cross sections (e.g., Swainson and Hammond, 2001) (Fig. 1). In some cases, the shape of the original ikaite crystal is preserved as a pseudomorph (e.g., Kaplan, 1978; Shearman and Smith, 1985; Kemper, 1987). The size of natural modern ikaite clusters and solitary crystals varies from ∼ 5 µm (Dieckmann et al., 2008) to ∼ 12 cm (Kodina et al., 2003). Glendonites display a number of internal structures, including (1) a visible core and rim with no apparent zonation; (2) core and alternating millimeter-scale rims, forming distinct zonation; and (3) homogeneous bodies from the center to the edges. Glendonites are characterized by a size range which mainly lies between 0.5 and 15–20 cm, with some of the largest specimens ranging up to 1 m in diameter or in length. Microglendonites are rarely reported; however, their size is similar to that of ikaite microcrystals, ranging from 5 to 10 µm (Oehlerich et al., 2013). Glendonite color varies from light yellow and whitish if weathered to dark brown.
Due to water removal during ikaite to anhydrous calcium carbonate transformation, glendonites are frequently porous and contain small pieces of adjacent sediment (such as sand grains, microfossils, clay particles, etc.) and several successive carbonate generations.
2.3 Isotopic composition of ikaite and glendonite
Stable carbon and oxygen isotopic values of glendonites and ikaites can encompass a broad range of values (Fig. 3). δ13C values of ikaite range from −42.7 ‰ to +8.3 ‰ PDB, while δ18O values range from −17 ‰ to +3.60 ‰ PDB (Dahl and Burchardt, 2006; Chaikovsky and Kadebskaya, 2014; Kodina et al., 2003; Krylov et al., 2015; Last et al., 2013; Lu et al., 2012, Stein and Smidt, 1985; Zabel and Schultz, 2001). The stable isotopic compositions of bulk glendonites and individual carbonate generations differ significantly. δ13C values of bulk glendonite samples range from −52.4 ‰ to +0.6 ‰ PDB, while δ18O values range from −16.6 ‰ to +4.8 ‰ PDB (Derkachev et al., 2007; Geptner et al., 1994, 2014; Qu et al., 2017; Willscroft et al., 2017; Vickers et al., 2018; Morales et al., 2017; Teichert and Luppold, 2013, among others). δ13C values of ikaite-derived calcite range from −27.7 ‰ to −14.3 ‰ PDB, while δ18O values range from −5.1 ‰ to +1.3 ‰ PDB (Frank et al., 2008). Lastly, δ13C values of secondary carbonate cements range from −20.2 ‰ to −3.9 ‰ PDB, while δ18O values range from −23.1 ‰ to −6.2 ‰ PDB (Frank et al., 2008; Vasileva et al., 2019; see Fig. 3). Based on the range of δ13C values, the source of carbon during ikaite crystallization and ikaite–glendonite transformation was derived from dissolved inorganic carbon (DIC), decaying organic matter, or methane seeping through underlying strata.
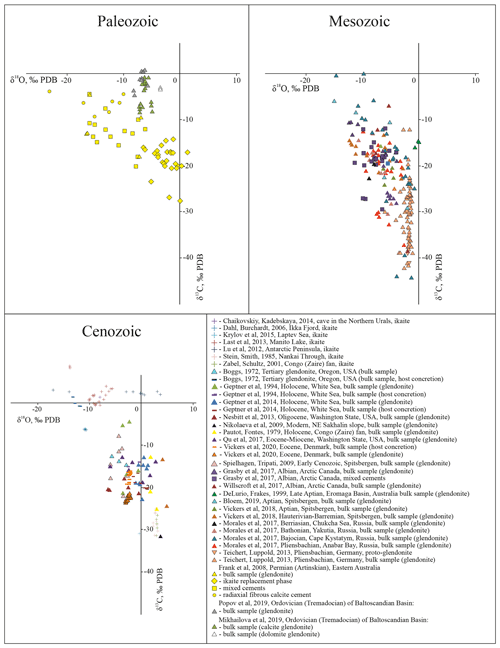
Figure 3Isotopic composition of glendonites (including different generations), host concretions, and ikaites for Paleozoic, Mesozoic, and Cenozoic samples.
Although Mesozoic and Cenozoic glendonites are enriched in Sr, they do not record primary ratios of seawater and show enrichment in 86Sr compared to coeval marine carbonates (Rogov et al., 2018; author's data).
3.1 Data mining
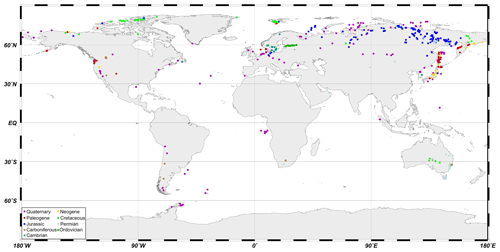
The authors have collected ikaite and glendonite specimens belonging to different stratigraphic intervals from numerous localities over the last 15 years. In addition to our specimens, we also studied glendonites from museum collections and analyzed information about glendonite and ikaite occurrences from numerous publications, using advanced online searches (including all of the different glendonite names mentioned above) along with library searches (see Supplement for details). The locations of all ikaite-/glendonite-bearing sites are shown in Fig. 4. The total number of records included in the database is 753, based on 376 references, our investigations, and communications by our colleagues.
3.2 Database contents
The database is provided as an Excel 2003 file (.xls). The main sheet of the database includes the following information: locality, age (substage/formation/Ma), modern coordinates, paleolatitudes (calculated using http://paleolatitude.org, last access: 21 December 2020; see van Hinsbergen et al., 2015), references, depositional settings, and host rock. Some data points lack information about paleolatitude (as they were derived from terrains with an uncertain geographic position) or host rock (in the case of some museum specimens). We aim to provide locality and coordinate information as precisely as possible. However, in some cases the available locality information was rather imprecise (for example, “basin of river `X”' or “mountain ridge `Y”'), although the effects of such imprecise location details on our analysis of (paleo)latitude distribution are considered insignificant. Paleolatitudes of Paleogene and Neogene glendonites from the northern margin of the Pacific cannot be determined using http://paleolatitude.org. We chose a similar paleolatitude to the present day for the Paleogene and Neogene based on Bazhenov et al. (1992), Harbert et al. (2000), and Kovalenko and Chernov (2003). Paleomagnetic data from Sakhalin (Weaver et al., 2003; Zharov, 2005) are more complicated and suggest a northward drift during the Cenozoic. The complex tectonic structure of the island precludes an accurate calculation of paleolatitude for each time slice and locality from Sakhalin. The database also includes stable carbon and oxygen isotope data and references.
4.1 Glendonite distribution in space and time
A significant irregularity in Phanerozoic glendonite distribution is
apparent in Fig. 4, which illustrates glendonite locations plotted on
present-day geography. However, when the paleolatitudes of glendonite
samples are taken into account, it becomes clear that they mainly formed in
high latitudes throughout the Phanerozoic (Fig. 5). Interestingly, most
glendonite occurrences have been reported from the Northern Hemisphere,
which is challenging to explain. Only Quaternary records of
ikaite and glendonite occurrences are fairly evenly distributed across the
cold-water environments of both the Northern Hemisphere and Southern Hemisphere and
within all ocean basins, including deep-water sites at low and
near-equatorial latitudes. Glendonite occurrences within the Arctic Ocean
(Kara Sea, Laptev Sea, Chukchi Sea) and the Sea of Okhotsk are more abundant
compared to other regions, which can be partially explained by sampling
bias, as these regions have been particularly actively studied in the
past few decades. In contrast to other geological periods, Neogene
glendonites are restricted to the northern margin of the Pacific, except for
a single reported occurrence in Arctic Canada. Glendonite occurrences are
particularly common at numerous localities along the western coast of the
Pacific Ocean, including from Japan, Sakhalin, and Kamchatka, where their
presence is used as a diagnostic feature for numerous formations and
members. Furthermore, one of these formations (Gennoishi Formation of
Sakhalin Island) is named after the Japanese name for glendonites
(gennoishi, ![]() ). Coeval glendonite records also occur along the eastern
Pacific coast, where they were first recognized by Dana (1849). Paleogene
glendonites typically occur in the same region as Neogene occurrences but
are also abundant in the North Atlantic area. North Atlantic occurrences
include giant glendonites from the Mors and Fur islands (Denmark), as well
as abundant Spitsbergen glendonites. It should be noted that the giant
glendonites from Denmark are mainly embedded in post-PETM (Paleocene–Eocene Thermal Maximum) rocks, but clumped
isotope data from the glendonites are indicative of near-freezing
temperatures (Nenning, 2017; Vickers et al., 2020).
). Coeval glendonite records also occur along the eastern
Pacific coast, where they were first recognized by Dana (1849). Paleogene
glendonites typically occur in the same region as Neogene occurrences but
are also abundant in the North Atlantic area. North Atlantic occurrences
include giant glendonites from the Mors and Fur islands (Denmark), as well
as abundant Spitsbergen glendonites. It should be noted that the giant
glendonites from Denmark are mainly embedded in post-PETM (Paleocene–Eocene Thermal Maximum) rocks, but clumped
isotope data from the glendonites are indicative of near-freezing
temperatures (Nenning, 2017; Vickers et al., 2020).
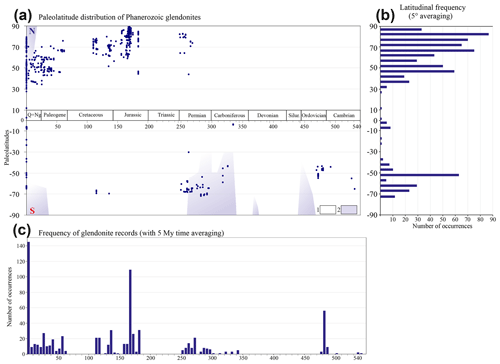
Figure 5Paleolatitudinal distribution of glendonite and ikaite records through time. (a) Paleolatitudinal occurrence; 1 – non-glacial intervals, 2 – glaciations (after Frakes et al., 2005); (b) latitudinal frequency of glendonite occurrences (with 5∘ averaging); (c) frequency of glendonite occurrence through time (with 5 Myr averaging).
The Late Cretaceous was characterized by a global greenhouse climate and lacks any glendonite occurrences. Lower Cretaceous glendonites are known from all stages and occurred in high latitudes of both the Northern Hemisphere and Southern Hemisphere. However, no occurrences are known from the most prominent warming event (early Aptian), and only a single occurrence has been reported from the Barremian. The paucity of Barremian glendonites may be related to the HALIP-induced regression in the Arctic and scarcity of marine deposits of this age in the high northern latitudes. Glendonites are particularly abundant within the Valanginian, Aptian, and Albian sediments of Svalbard (Kemper, Schmitz, 1981; Vickers et al., 2018) and Arctic Canada (Herrle et al., 2015), along with the Aptian of Australia (de Lurio, Frakes, 1999). Berriasian glendonites, which mark the initiation of the Early Cretaceous cold interval, are rare and mainly described from Siberia (Rogov et al., 2017).
Peak glendonite abundance, comparable to that of the Quaternary, occurs during the Middle Jurassic, but nearly all occurrences of this age have been described from northern Eurasia, with no occurrences in the Southern Hemisphere. The numerous Middle Jurassic glendonite occurrences coincide with long-term cooling of the Arctic Ocean, primarily instigated by changes in oceanic circulation following closure of the Viking corridor (Korte et al., 2015). The Late Jurassic is characterized by a significant decrease in glendonite abundance towards the end of this time interval. Lower Jurassic glendonite occurrences are restricted to the upper Pliensbachian and a few in the upper Toarcian.
No glendonites have been confidently identified from the Triassic period. Only two suspicious reports of pseudomorphs resembling glendonites from low-latitude lagoonal environments were mentioned by Kaplan (1979), based on reports by van Houten (1965) and Kostecka (1975).
Permian glendonite occurrences display a similar distribution to Lower Cretaceous occurrences. They are also known from both the Northern Hemisphere and Southern Hemisphere, but they are especially abundant in Tasmania and Australia (Selleck et al., 2007), while in the Northern Hemisphere they are mainly found in northeast Asia (Biakov et al., 2013), accompanied by rare findings in the rest of Siberia and Novaya Zemlya. Possible glendonites from Turkey and Saudi Arabia are poorly described.
Carboniferous glendonites are uncommon and mainly known from the Upper Carboniferous of Gondwana. In addition, glendonites were also reported from low-latitude carbonate deposits of Alberta, Canada (Brandley, Krause, 1994).
There is a notable absence of glendonite occurrences for a prolonged period of time below the Carboniferous, spanning the Devonian, Silurian, and Middle–Upper Ordovician. Lower Ordovician glendonites have only recently been discovered and described (Popov et al., 2019; Mikhailova et al., 2019), as until recently, they were known as a kind of “anthraconite” and were not considered to be glendonites. Their findings are restricted to Baltoscandia. Cambrian glendonites are less frequent but are mainly known from the same region and same facies (black shales) as the Lower Ordovician glendonites. A few Cambrian glendonites were also found in sandstones, and a single occurrence is known from outside the Baltic region in South Korea (Chon, 2018).
4.2 Glendonites as paleoenvironmental indicators
Over the past few decades, glendonites have been used as a proxy for cold-water environments and/or cooling towards glaciation events (Kaplan, 1980; Kemper and Schmitz, 1981; Suess et al., 1982; De Lurio and Frakes, 1999; Price, 1999; Swainson and Hammond, 2001; Selleck et al., 2007; Rogov et al., 2017, among others). This interpretation is grounded on the discovery of ikaite precipitation during early diagenesis at low temperatures in Holocene–Pleistocene marine sediments, confined to layers with elevated carbonate alkalinity, high pH, and high concentrations of dissolved phosphate (e.g., Kodina et al., 2003; Greinert and Derkachev, 2004; Zhou et al., 2015). The increase in alkalinity, which promotes crystallization of ikaite, is caused by biogeochemical processes in marine sediments, such as organoclastic sulfate reduction and/or anaerobic oxidation of methane (Schubert et al., 1997; Suess et al., 1982; Kodina et al., 2003; Krylov et al., 2015; Lu et al., 2012; Zabel and Schulz, 2001).
However, experiment results in the last few years suggest at least short-term ikaite stability at much higher temperatures, up to 30–35 ∘C (Clarkson et al., 1992; Rodríguez-Ruiz et al., 2014; Purgstaller et al., 2017; Stockmann et al., 2018; Tollefsen et al., 2020). Ikaite stability at high temperatures had only previously been demonstrated under high pressures (van Valkenberg et al., 1971). It is important to note that high-temperature experiments were only conducted on ikaite microcrystal precipitates, and there is currently no evidence for stability of “typical” centimeter- to decimeter-sized ikaite crystals and ikaite clusters at similarly high temperatures. Besides that, synthetic ikaites are mainly synthesized in simplified systems, whereas natural ikaites may contain some structural impurities, which could sufficiently influence their field of stability.
Our analysis of glendonite distribution through space and time has revealed a complex relationship between climate change and glendonite occurrence. On the one hand, nearly all glendonite findings known to date (except for a few doubtful ones) are associated with cold-water environments and are usually found in sediments with high-latitude low-diversity faunas and dropstones (e.g., Price, 1999). On the other hand, no glendonites have been discovered from two prominent glacial intervals in Earth's history (the Late Ordovician and Late Devonian), and their pre-Quaternary occurrences are very irregular, changing to the Southern Hemisphere in early Paleozoic to mostly Northern Hemisphere in Mesozoic and Cenozoic. However, the roles of additional factors influencing ikaite precipitation remain unclear.
The .xls file containing this database is available on Zenodo https://doi.org/10.5281/zenodo.4386335 (Rogov et al., 2020). New versions of the database will also be published via Zenodo. The most recent version will always be accessible via https://doi.org/10.5281/zenodo.4289834 (Rogov et al., 2020), through direct hyperlink (http://jurassic.ru/_pdf/glendonites_database.xls, last access: 21 December 2021) or can be requested directly from the first author of this paper.
Our new reference glendonite and ikaite database can be used to show that glendonites typically occur in cold-water environments throughout the Phanerozoic, but their distribution is highly irregular in space and time. Although there is no evidence for glendonite formation in warm-water settings, not all of Earth's major glaciations and other cooling events are associated with glendonite occurrences. Significant irregularity also occurs in spatial glendonite distribution throughout the Phanerozoic. Quaternary glendonites and ikaites have been described from all ocean basins of the world, while pre-Quaternary glendonites have not been found in many regions which would appear to be suitable for ikaite precipitation.
The supplement related to this article is available online at: https://doi.org/10.5194/essd-13-343-2021-supplement.
MR designed the study and collected data on stratigraphic and geographic distribution of glendonite. VE did the overall editing and supervision. OV collected data on morphology and mineralogical composition. KV and KM were responsible for data on isotopic composition, petrography, and cathodoluminescence. AK provided data on ikaite distribution and diagenesis. All authors discussed the results and participated in preparation of the paper.
The authors declare that they have no conflict of interest.
The authors thank all colleagues who provided data and samples for this research. We thank Aleksei R. Sokolov (TsNIGR Museum, St. Petersburg, Russia), Pavel Yu. Plechov (Fersman Mineralogical Museum of RAS, Moscow, Russia), and Iraida A. Starodubtseva (Vernadsky Geological Museum, Moscow, Russia) for donating specimens of glendonites used in this study. Very important glendonite specimens from the Barents Sea shelf were provided by Marina N. Rudenko (VNIIOKeangeologiya, St. Petersburg, Russia) and Anna S. Strezh (VNIGNI, Moscow, Russia). Information about glendonite occurrences (mainly stratigraphic data and coordinates of localities) was received from Aleksandr S. Biakov (North-East Interdisciplinary Scientific Research Institute, FEB RAS, Magadan, Russia); Andrey V. Dronov, Victoria V. Kostyleva, Aleksandr B. Kuzmichev, Tatiana N. Palechek, Boris G. Pokrovsky, Marianna I. Tuchkova, and Victor A. Zakharov (Geological Institute of RAS, Moscow, Russia); Valery M. Gorozhanin (Institute of Geology of USC, 5 Ufa, Russia); Evgeny A. Gusev (VNIIOkeangeologiya, St. Petersburg, Russia); Anna A. Feodorova (Geologorazvedka, St. Petersburg, Russia); Georgy S. Iskul (VSEGEI, St. Petersburg, Russia); Malte Jochmann (UNIS, Svalbard, Norway); Vladimir A. Marinov (Tyumen Oil Scientific Center, Tyumen, Russia); Chloé Morales (INGEN, Dijon, France); Aleksandr E. Igolnikov, Boris L. Nikitenko, Lyudmila G. Vakulenko, and Petr A. Yan (Institute of Petroleum Geology and Geophysics SB RAS, Novosibirsk, Russia); Aleksandr V. Osinzev (Arabica Speleological Club, Irkutsk, Russia); Simon Schneider (CASP, Cambridge, UK); Bo Pagh Schulz (Museum Salling, Fur Museum, Fur, Denmark); Renat B. Shakirov and Maksim G. Valitov (Pacific Oceanological Institute FEB RAS, Vladivostok, Russia); Irina A. Tarasenko (Far East Geological Institute, Vladivostok, Russia); Ursula Toom (Tallinn University of Technology, Tallinn, Estonia); and Valery R. Trofimov (Krasnoyarsk, Russia). Glendonite records mentioned in unpublished reports by the Rosneft Oil Company (Russia) are used with permission from headquarters of LLC RN-Shelf Arktika. Special thanks are due to James Barnet (Camborne School of Mines) for editing the English of a previous version of the paper. We are very grateful for the constructive reviews of the paper by Madeleine Vickers and the anonymous reviewer.
This research has been supported by the Russian Foundation for Basic Research (grant no. 20-35-70012) (to Victoria Ershova, Oleg Vereshchagin, Kseniia Vasileva, and Kseniia Mikhailova). Aleksei Krylov received financial support from the Russian Science Foundation (grant no. 19-17-00226).
This paper was edited by Kirsten Elger and reviewed by Madeleine Vickers and one anonymous referee.
Astakhova, N. V. and Sorochinskaya, A. V.: Authigenic carbonates of the upper Pleistocene-Holocene deposits in the Northwest Pacific Marginal Seas, Russ. J. Pacific Geol., 16, 65–78, 2000.
Bazhenov, M. L., Burtman, V. S., Krezhovshikh, O. A. and Shapiro, M. N.: Paleomagnetism of Paleogene rocks of the Central–East Kamchatka and Komanorsky Islands: tectonic implications, Tectonophysics, 201, 157–173, https://doi.org/10.1016/0040-1951(92)90181-5, 1992.
Biakov, A. S., Goryachev, N. A., Davydov, V. I., and Vedernikov, I. L.: The first finds of glendonite in permian deposits of the North Okhotsk Region, Northeastern Asia, Dokl. Earth Sci., 451, 716–718, https://doi.org/10.1134/S1028334X13070210, 2013.
Bischoff, J. L., Stine, S., Rosenbauer, R. J., Fitzpatrick, J. A., and Stafford Jr., T. W.: Ikaite precipitation by mixing of shoreline springs and lake water, Mono Lake, California, USA, Geochim. Cosmochim. Ac., 57, 3855–3865, https://doi.org/10.1016/0016-7037(93)90339-X, 1993.
Bloem, M.: The Petrology and Inorganic Geochemistry of Glendonites: Insights into the enigmatic ikaite-to-glendonite transformation using a detailed combinative approach, Master thesis, Utrecht University, Netherlands, 75 pp., 2019.
Boggs Jr., S.: Petrography and geochemistry of rhombic, calcite pseudomorphs from mid Tertiary mudstones of the Pacific Northwest, U.S.A, Sedimentology, 19, 219–235, https://doi.org/10.1111/j.1365-3091.1972.tb00022.x, 1972.
Brandley, R. T. and Krause, F. F.: Thinolite type pseudomorphs after ikaite: Indicators of coldwater on the subequatorial western margin of Lower Carboniferous North America, GSPG Memoir, 17, 333–334, https://doi.org/10.11575/PRISM/30223, 1994.
Brooks, R., Clark, L. M., and Thurston, E. F.: Calcium carbonate and its hydrates, Philos. T. Roy. Soc. Lond. A, 243, 145–167, https://doi.org/10.1098/rsta.1950.0016, 1950.
Chaikovskiy, I. I. and Kadebskaya, O. I.: Mineral bodies of the Eranka cave in the Northern Urals, Probl. Mineral. Petrogr. Metal., 17, 92–107, 2014 (in Russian).
Chon, S.-J.: First report of glendonite in the uppermost Cambrian, in GSA Annual Meeting, Indiana, USA, 4–7 November 2018, 265–3, https://doi.org/10.1130/abs/2018am-321990, 2018.
Clarkson, J. R., Price, T. J., and Adams, C. J.: Role of metastable phases in the spontaneous precipitation of calcium carbonate, J. Chem. Soc. Faraday Trans., 88, 243–249, https://doi.org/10.1039/ft9928800243, 1992.
Council, T. C. and Bennett, P. C.: Geochemistry of ikaite formation at Mono Lake, California: Implications for the origin of tufa mounds, Geology, 21, 971–974, https://doi.org/10.1130/0091-7613(1993)021<0971:GOIFAM>2.3.CO;2, 1993.
Dahl, K. and Buchardt, B.: Monohydrocalcite in the Arctic Ikka Fjord, SW Greenland: first reported marine occurrence, SEPM, 76, 460–471, https://doi.org/10.2110/jsr.2006.035, 2006.
Dana, J. D.: United States Exploring Expedition. During the years 1838, 1839, 1840, 1841, 1842. Under the command of Charles Wilkes, 735 pp., available at: https://www.biodiversitylibrary.org/item/135966#page/11/mode/1up (last access: 8 February 2021), 1849.
David, T. W. E., Taylor, T. G., Woolnough, W. G., and Foxall, H. G.: Occurrence of the pseudomorph glendonites in New South Wales, Records of the Geological Survey of New South Wales, 8, 162–179, 1905.
Derkachev, A. N., Nikolaeva, N. A., Mozherovsky, A. V., Grigor'eva, T. N., Ivanova, E. D., Pletnev, S. P., Barinov, N. N., and Chubarov, V. M.: Mineralogical and geochemical indicators of anoxic sedimentation conditions in local depressions within the Sea of Okhotsk in the Late Pleistocene-Holocene, Russ. J. Pac. Geol., 1, 203–229, https://doi.org/10.1134/S1819714007030013, 2007.
Dieckmann, G. S., Nehrke, G., Papadimitriou, S., Gottlicher, J., Steininger, R., Kennedy, H., Wolf-Gladrow, D., and Thomas, D. N.: Calcium carbonate as ikaite crystals in Antarctic sea ice, Geophys. Res. Lett., 35, L08501, https://doi.org/10.1029/2008GL033540, 2008.
Fairchild, I. J., Fleming, E. J., Bao, H., Benn, D. I., Boomer, I., Dublyansky, Y. V., Halverson, G. P., Hambrey, M. J., Hendy, C., Mcmillan, E. A., Spotl, C., Stevenson C. T. E., and Wynn, P. M.: Continental carbonate facies of a Neoproterozoic panglaciation, north-east Svalbard, Sedimentology, 63, 443–497, https://doi.org/10.1111/sed.12252, 2016.
Fink, H. G., Strasser M., Römer, M., Kölling, M., Ikehara, K., Kanamatsu, T., Dinten, D., Kioka, A., Fujiwara, T., Kawamura, K., Kodaira, S., and Wefer, G.: Evidence for Mass Transport Deposits at the IODP JFAST-Site in the Japan Trench, Adv. Nat. Technol. Haz., 37, 33–43, https://doi.org/10.1007/978-3-319-00972-8_4, 2014.
Frakes, L. A., Francis, J. E., and Syktus, J. I.: Climate Modes of the Phanerozoic, Cambridge Univ. Press, Cambridge, 2005.
Frank, T. D., Thomas, S. G., and Fielding, C. R.: On using carbon and oxygen isotope data from glendonites as paleoenvironmental proxies: a case study from the Permian system of eastern Australia, J. Sediment. Res., 78, 713–723. https://doi.org/10.2110/jsr.2008.081, 2008.
Freisleben, J. K.: Ueber einige interessante Vorkommnisse im Schlotztenleimen (Alluvialthon) bey Obersdorf, ohnweit Sangerhausen, Isis von Oken, 20, 334–337, 1827 (in German).
Geptner, A. R., Pokrovsky, B. G., Sadchikova, T. A., Sulerzhitsky, L. D., and Chernyakhovsky, A. G.: Local carbonatization of the White Sea sediments (concept of microbiological formation), Lithol. Mineral. Resour., 5, 3–22, 1994.
Geptner, A. R., Vetoshkina, O. S., and Petrova V. V.: New data on the composition of stable isotopes in glendonites of the White Sea and their genesis, Lithol. Mineral. Resour., 49, 473–490, https://doi.org/10.1134/S0024490214060042, 2014.
Grasby, S. E., McCune, G. E., Beauchamp, B., and Galloway, J. M. Lower Cretaceous cold snaps led to widespread glendonite occurrences in the Sverdrup Basin, Canadian High Arctic, GSA Bulletin, 129, 771–787, https://doi.org/10.1130/B31600.1, 2017.
Greinert, J. and Derkachev, A.: Glendonites and methane-derived Mg-calcites in the Sea of Okhotsk, Eastern Siberia: implications of a venting-related ikaite/glendonite formation, Mar. Geol., 204, 129–144, https://doi.org/10.1016/S0025-3227(03)00354-2, 2004.
Harbert, W., Kepezhinskas, P., Krylov, K., Grigoriev, V., Sokolov, S., and Aleksuitin, M.: Paleomagnetism and Tectonics of the Kamchatka Region, Northeastern Russia: Implications for Development and Evolution of the Northwest Pacific Basin, Polarforschung, 68, 297–308, 2000.
Herrle, J. O., Schroder-Adams, C., Davis, W., Pugh, A. T., Galloway, J. M., and Fath, J.: Mid-Cretaceous High Arctic stratigraphy, climate, and oceanic anoxic events, Geology, 43, 403–406, https://doi.org/10.1130/G36439.1, 2015.
Hesse, K.-F., Kuppers, H., and Suess, E.: Refinement of the structure of Ikaite, CaCO3*6H2O, Z. Kristallographie, 231, 227–231, https://doi.org/10.1524/zkri.1983.163.3-4.227, 1983.
Hu, Y. B., Wolf-Gladrow, D. A., Dieckmann, G. S., Völker, C., and Nehrke, G.: A laboratory study of ikaite (CaCO3· 6H2O) precipitation as a function of pH, salinity, temperature and phosphate concentration, Mar. Chem., 162, 10–18, https://doi.org/10.1016/j.marchem.2014.02.003, 2014.
Hu, Y. B., Wolthers, M., Wolf-Gladrow, D. A., and Nehrke, G.: Effect of pH and phosphate on calcium carbonate polymorphs precipitated at near-freezing temperature, Cryst. Growth Des., 15, 1596–1601, https://doi.org/10.1021/cg500829p, 2015.
Huggett, J. M., Schultz, B. P., Shearman, J., and Smith, A. J.: The petrology of ikaite pseudomorphs and their diagenesis, Proc. Geol. Assoc., 116, 207–220, https://doi.org/10.1016/S0016-7878(05)80042-2, 2005.
Ito, T.: Ikaite from cold spring water at Shiowakka, Hokkaido, Japan, J. Mineral. Petrol. Econ. Geol., 91, 209–219, https://doi.org/10.2465/ganko.91.209, 1996.
Ito, T.: Factors controlling the transformation of natural ikaite from Shiowakka, Japan, Geochem. J., 32, 267–273, https://doi.org/10.2343/geochemj.32.267, 1998.
Ito, T., Matsubara, S., and Miyawaki, R.: Vaterite after ikaite in carbonate sediment, J. Petrol. Mineral. Econ. Geol., 94, 176–182, https://doi.org/10.2465/ganko.94.176, 1999.
Kaplan, M. E.: Calcite pseudomorphoses from the Jurassic and lower Cretaceous deposits of northern East Siberia, Sov. Geol. Geophys., 12, 62–70, 1978 (in Russian).
Kaplan, M. E.: Calcite pseudomorphs (pseudogaylussite, jarrowite, thinolite, glendonite, gennoishi, White Sea hornlets) in sedimentary rocks, Review of major localities, VINITI, 39 pp., 1979 (in Russian).
Kaplan, M. E.: Calcite pseudomorphs (pseudogaylussite, jarrowite, thinolite, glendonite, gennoishi, White Sea hornlets) in sedimentary rocks: origins of the pseudomorphs, Lithol. Miner. Resour., 14, 623–636, 1980.
Kemper, E. and Schmitz, H. H.: Stellate nodules from the upper Deer Bay formation (Valanginian) of Arctic Canada, Geol. Surv. Can. Pap., 75, 109–119, https://doi.org/10.4095/103040, 1975.
Kemper, E. and Schmitz, H. H.: Glendonite – Indikatoren des polarmarinen Ablagerungsmilieus, Geol. Rundsch., 70, 759–773, https://doi.org/10.1007/bf01822149, 1981.
Kemper, E.: Das Klima der Kreide-Zeit, Geologisches Jahrbuch Reiche A, 96, 5–185, 1987 (in German).
Kodina, L. A., Tokarev, V. G., Vlasova, L. N., and Korobeinik, G. S.: Contribution of biogenic methane to ikaite formation in the Kara Sea: evidence from the stable carbon isotope geochemistry, in: Siberian River Run-off in the Kara Sea: Characterisation, Quantification, Variability, and Environmental Significance, edited by: Stein, R., Fahl, K., Fütterer, D. K., Galimov, E. M., and Stepanets, O. V., 488 pp., Proceedings in Marine Sciences, Elsevier, Amsterdam, 6, 349–374, 2003.
Korte, C., Hesselbo, S. P., Ullmann, C. V., Dietl, G., Ruhl, M., Schweigert, G., and Thibault, N.: Jurassic climate mode governed by ocean gateway, Nat. Commun., 6, 10015, https://doi.org/10.1038/ncomms10015, 2015.
Kostecka, A.: Calcite paramorphs in the aragonite concretions, Ann. Soc. Geol. Pol., 42, 289–296, 1975.
Kovalenko, D. V. and Chernov, Y. Y.: Paleomagnetism and tectonic evolution of Kamchatka and Southern Koryakia, Russ. J. Pac. Geol., 22, 48–73, 2003.
Krylov, A. A., Logvina, E. A., Matveeva, T. M., Prasolov, E. M., Sapega, V. F., Demidova, A. L. and Radchenko, M. S.: Ikaite (CaCO3*6H2O) in bottom sediments of the Laptev Sea and the role of anaerobic methane oxidation in this mineral-forming process, Proc. Russ. Mineral. Soc., 4, 61–75, 2015 (in Russian).
Larsen, D.: Origin and paleoenvironmental significance of calcite pseudomorphs after ikaite in the Oligocene Creede Formation, Colorado, J. Sediment. Res., 64, 593–603, https://doi.org/10.1306/D4267E1A-2B26-11D7-8648000102C1865D, 1994.
Last, F. M. and Last, W. M.: Lacustrine carbonates of the northern Great Plains of Canada, Sediment. Geol., 277–278, 1–31, https://doi.org/10.1016/j.sedgeo.2012.07.011, 2012.
Last, F. M., Last, W. M., Fayek, M., and Halden, N. M.: Occurrence and significance of a cold-water carbonate pseudomorph in microbialites from a saline lake, J. Paleolimnol., 50, 505–517, https://doi.org/10.1007/s10933-013-9742-6, 2013.
Lennie, A. R., Tang, C. C., and Thompson, S. P.: The structure and thermal expansion behaviour of ikaite, CaCO3*6H2O, from T=114 to T = 293 K, Mineral. Mag., 68, 135–146, https://doi.org/10.1180/0026461046810176, 2004.
Loog, A.: On the geochemistry of postsedimentary mineral formation in the Tremadocian graptolithic argillites of North Estonia, Acta et Commentationes Universitatis Tartuensis, 527, 44–50, 1980 (in Russian).
Lu, Z., Rickaby, R. E., Kennedy, H., Kennedy, P., Pancost, R. D., Shaw, S., Lennie, A., Wellner, J. and Anderson, J. B.: An ikaite record of late Holocene climate at the Antarctic Peninsula, Earth Planet. Sc. Lett., 325, 108–115, https://doi.org/10.1016/j.epsl.2012.01.036, 2012.
de Lurio, J. and Frakes, L. A.: Glendonites as a paleoenvironmental tool: implications for early Cretaceous high latitude climates in Australia – its composition and evolution, Geochim. Cosmochim. Ac., 63, 1039–1048, https://doi.org/10.1016/S0016-7037(99)00019-8, 1999.
Marland, G.: The stability of CaCO3*6H2O, Geochim. Cosmochim. Ac., 39, 83–91, https://doi.org/10.1016/0016-7037(75)90186-6, 1975.
McLachlan, I. R., Tsikos, H., and Cairncross, B.: Glendonites (pseudomorphs after ikaite) in late carboniferous Marine Dwyka beds in Southern Africa, S. Afr. J. Geol., 104, 265–272, https://doi.org/10.2113/1040265, 2001.
Mikhailova, K., Vasileva, K., Fedorov, P., Ershova, V., Vereshchagin, O., Rogov, M., and Pokrovsky, B.: Glendonite-Like Carbonate Aggregates from the Lower Ordovician Koporye Formation (Russian Part of the Baltic Klint): Detailed Mineralogical and Geochemical Data and Paleogeographic Implications, Minerals, 9, 524, https://doi.org/10.3390/min9090524, 2019.
Morales, C., Rogov, M., Wierzbowski, H., Ershova, V., Suan, G., Adatte, T., Föllmi, K. B., Tegelaar, E., Reichart, G. J., de Lange, G. J., and Middelburg, J. J.: Glendonites track methane seepage in Mesozoic polar seas, Geology, 45, 503–506, https://doi.org/10.1130/G38967.1. 2017.
Nenning, F.: Mega-glendonites in the Early Eocene Fur Formation. Unraveling paleoenvironmental conditions in the Danish Basin and their influence on glendonite formation, PhD thesis, Westfälischen Wilhelms-Universität Münster, 177 pp., 2017.
Nesbitt, E. A., Martin, R. A., and Campbell, K. A.: New records of Oligocene diffuse hydrocarbon seeps, northern Cascadia margin, Palaeogeogr. Palaeoclimatol. Palaeoecol., 390, 116–129, https://doi.org/10.1016/j.palaeo.2013.05.001, 2013.
Nikolaeva, N. A., Derkachev, A. N., and Obzhirov, A. I.: Characteristic features of the occurrence of gas-fluid emanations on the northeastern slope of Sakhalin Island, Sea of Okhotsk, Russ. J. Pac. Geol., 3, 234–248, https://doi.org/10.1134/S181971400903004X, 2009.
Oehlerich, M., Mayr, C., Griesshaber, E., Lucke, A., Oeckler, O. M., Ohlendorf, C., Schmahl, W. W., and Zolitschka, B.: Ikaite precipitation in a lacustrine environment–implications for palaeoclimatic studies using carbonates from Laguna Potrok Aike (Patagonia, Argentina), Quaternary Sci. Rev., 71, 46–53, https://doi.org/10.1016/j.quascirev.2012.05.024, 2013.
Oehlerich, M., Mayr, C., Gussone, N., Hahn, A., Holzl, S., Lucke, A., Ohlendorf, C., Rummael, S., Teichert, B. M. A., and Zolitschka, B.: Lateglacial and Holocene climatic changes in south-eastern Patagonia inferred from carbonate isotope records of Laguna Potrok Aike (Argentina), Quaternary Sci. Rev., 114, 189–202, https://doi.org/10.1016/j.quascirev.2015.02.006, 2015.
Pander, C. H.: Beiträge zur Geognosie des Russischen Reiches, Karl Kray, St.Petersburg, 1830.
Pauly, H.: “Ikaite”, a new mineral from Greenland, Arctic, 16, 263–264, https://doi.org/10.14430/arctic3545, 1963.
Pautot, G. and Fontes, J.-C.: Gypsum-calcite rosettes in sediments from the Congo deep-sea fan, Oceanol. Ac., 2, 329–334, 1979.
Popov, L. E., Alvaro, J. J., Holmer, L. E., Bauert, L. E., Pour, M. G., Dronov, A. V., Lehnert, O., Hints, O., Mannik, P., Zhang, Z., and Zhang, Z.: Glendonite occurrences in the Tremadocian of Baltica: first Early Palaeozoic evidence of massive ikaite precipitation at temperate latitudes, Sci. Rep.-UK, 9, 1–10, https://doi.org/10.1038/s41598-019-43707-4, 2019.
Price, G. D.: The evidence and implications of polar ice during the Mesozoic, Earth-Sci. Rev., 48, 183–210, https://doi.org/10.1016/S0012-8252(99)00048-3, 1999.
Purgstaller, B., Dietzel, M., Baldermann, A., and Mavromatis, V.: Control of temperature and aqueous Mg2+/Ca2+ ratio on the (trans-)formation of ikaite, Geochim. Cosmochim. Ac., 217, 128–143, https://doi.org/10.1016/j.gca.2017.08.016, 2017.
Qu, Y., Teichert, B. M. A., Birgel, D., Goedert, J. L., and Peckmann, J.: The prominent role of bacterial sulfate reduction in the formation of glendonite: a case study from Paleogene marine strata of western Washington State, Facies, 63, 10, https://doi.org/10.1007/s10347-017-0492-1, 2017.
Rodriguez-Ruiz, I., Veesler, S., Gomez-Morales, J., Delgado-Lopez, J. M., Grauby, O., Hammadi, Z., Candoni, N., and Garcia-Ruiz, J. M.: Transient Calcium Carbonate Hexahydrate (Ikaite) Nucleated and Stabilized in Confined Nano- and Picovolumes, Cryst. Growth. Des., 4, 792–802, https://doi.org/10.1021/cg401672v, 2014.
Rogala, B., James, N. P., and Reid, C. M.: Deposition of polar carbonates during interglacial highstands on an Early Permian shelf, Tasmania, J. Sediment. Res., 77, 587–606, https://doi.org/10.2110/jsr.2007.060, 2007.
Rogov, M. A., Kuznetsov, A. B., Konstantinova, G. V., and Turchenko, T. L.: The Strontium Isotopic Composition in Glendonites of the Middle Jurassic in Northern Siberia, Dokl. Earth Sci., 482, 1168–1172, https://doi.org/10.1134/S1028334X1809009X, 2018.
Rogov, M. A., Ershova, V. B., Shchepetova, E. V., Zakharov, V. A., Pokrovsky, B. G., and Khudoley, A. K.: Earliest Cretaceous (late Berriasian) glendonites from Northeast Siberia revise the timing of initiation of transient Early Cretaceous cooling in the high latitudes, Cretac. Res., 71, 102–112, https://doi.org/10.1016/j.cretres.2016.11.011, 2017.
Rogov, M., Vasileva, K., Mikhailova, K., and Ershova, V.: Database of global glendonite and ikaite records throughout the Phanerozoic, Zenodo, https://doi.org/10.5281/zenodo.4386335, 2020.
Rysgaard, S., Søgaard, D. H., Cooper, M., Pućko, M., Lennert, K., Papakyriakou, T. N., Wang, F., Geilfus, N. X., Glud, R. N., Ehn, J., McGinnis, D. F., Attard, K., Sievers, J., Deming, J. W., and Barber, D.: Ikaite crystal distribution in winter sea ice and implications for CO2 system dynamics, The Cryosphere, 7, 707–718, https://doi.org/10.5194/tc-7-707-2013, 2013.
Rysgaard, S., Wang, F., Galley, R. J., Grimm, R., Notz, D., Lemes, M., Geilfus, N.-X., Chaulk, A., Hare, A. A., Crabeck, O., Else, B. G. T., Campbell, K., Sørensen, L. L., Sievers, J., and Papakyriakou, T.: Temporal dynamics of ikaite in experimental sea ice, The Cryosphere, 8, 1469–1478, https://doi.org/10.5194/tc-8-1469-2014, 2014.
Schlitzer, R.: Ocean Data View: available at: https://odv.awi.de, last access: 2 August 2020.
Schubert, C. J., Nürnberg, D., Scheele, N., Pauer, F., and Kriews, M.: 13C isotope depletion in ikaite crystals: evidence for methane release from the Siberian shelves?, Geo-Mar. Lett., 17, 169–174, https://doi.org/10.1007/s003670050023, 1997.
Selleck, B. W., Carr, P. F., and Jones, B. G.: A review and synthesis of glendonites (pseudomorphs after ikaite) with new data: assessing applicability as recorders of ancient coldwater conditions, J. Sediment. Res., 77, 980–991, https://doi.org/10.2110/jsr.2007.087, 2007.
Shahar, A., Bassett, W. A., Mao, H.-K., Chou, I.-M., and Mao, W.: The stability and Raman spectra of ikaite, CaCO3•6H2O, at high pressure and temperature, Am. Mineral., 90, 1835–1839, https://doi.org/10.2138/am.2005.1783, 2005.
Shaikh, A. M.: A new crystal growth form of vaterite, CaCO3, J. Appl. Crystallogr., 23, 263–265, https://doi.org/10.1107/S0021889890002485, 1990.
Shearman, D. J. and Smith, A. J.: Ikaite, the parent mineral of jarrowite-type pseudomorphs, Proc. Geol. Assoc., 96, 305–314, doi.org/10.1016/S0016-7878(85)80019-5, 1985.
Sokolov, D.: On White Sea mineral, Mining journal (Gorny zhurnal), 6, 117–120, 1825 (in Russian).
Spielhagen, R. F. and Tripati, A.: Evidence from Svalbard for near-freezing temperatures and climate oscillations in the Arctic during the Paleocene and Eocene, Palaeogeogr. Palaeoclim., 278, 48–56, https://doi.org/10.1016/j.palaeo.2009.04.012, 2009.
Stein, C. L. and Smith, A. J.: Authigenic carbonate nodules in the Nankai Trough, Site 583, Init. Repts. DSDP., 87, 659–668, https://doi.org/10.2973/dsdp.proc.87.115.1986, 1986.
Stockmann, G., Tollefsen, E., Skelton, A., Brüchert, V., Balic-Zunic, T., Langhof, J., Skogby, H., and Karlsson, A.: Control of a calcite inhibitor (phosphate) and temperature on ikaite precipitation in Ikka Fjord, southwest Greenland, Appl. Geochem., 89, 11–22, https://doi.org/10.1016/j.apgeochem.2017.11.005, 2018.
Suess, E., Balzer, W., Hesse, K.-F., Muller, P. J., Ungeree, C. A., and Wefer, G.: Calcium Carbonate Hexahydrate from Organic-Rich Sediments of the Antarctic Shelf: Precursors of Glendonites, Science, 216, 1128–1131, https://doi.org/10.1126/science.216.4550.1128, 1982.
Swainson, I. P. and Hammond, R. P.: Ikaite, CaCO3 ⋅ 6H2O: Cold comfort for glendonites as paleothermometers, Am. Mineral., 86, 1530–1533, https://doi.org/10.2138/am-2001-11-1223, 2001.
Tang, C. C., Thompson, S., Parker, J., and Lennie, A. R.: The ikaite-to-vaterite transformation: New evidence from diffraction and imaging, J. Appl. Crystallogr., 42, 225–233, https://doi.org/10.1107/S0021889809005810, 2009.
Teichert, B. M. A. and Luppold, F. W.: Glendonites from an Early Jurassic methane seep – Climate or methane indicators?, Palaeogeogr. Palaeoclim., 390, 81–93, https://doi.org/10.1016/j.palaeo.2013.03.001, 2013.
Tollefsen, E., Balic-Zunic, T., Morth, C.-M., Bruchert, V., Choo, C., Skelton, L., and Skelton, A.: Ikaite nucleation at 35 ∘C challenges the use of glendonite as a paleotemperature indicator, Sci. Rep.-UK, 10, 8141, https://doi.org/10.1038/s41598-020-64751-5, 2020.
van Hinsbergen, D. J. J., de Groot, L. V., van Schaik, S. J., Spakman, W., Bijl, P. K., Sluijs, A., Langereis, C. G., and Brinkhuis, H.: A Paleolatitude Calculator for Paleoclimate Studies, Plos One, 10, e0126946, https://doi.org/10.1371/journal.pone.0126946, 2015.
van Houten, F. B.: Crystal casts in Upper Triassic Lockatong and Brunswick Formations, Sedimentology, 4, 301–313, https://doi.org/10.1111/j.1365-3091.1965.tb01553.x, 1965.
van Valkenberg, A., Mao, H. K., and Bell, P. M.: Ikaite (CaCO3x6H2O), a phase more stable than calcite and aragonite (CaCO3) at high water pressure, Carnegie Inst. Wash. Yearb., 70, 237–238, 1971.
Vasileva, K. Y., Rogov, M. A., Ershova, V. B. and Pokrovsky, B G.: New results of stable isotope and petrographic studies of Jurassic glendonites from Siberia, GFF, 141, 225–232, https://doi.org/10.1080/11035897.2019.1641549, 2019.
Vickers, M., Watkinson, M., Price, G. D., and Jerret, R.: An improved model for the ikaite-glendonite transformation: evidence from the Lower Cretaceous of Spitsbergen, Svalbard, Nor. J. Geol., 98, 1–15, https://doi.org/10.17850/njg98-1-01, 2018.
Vickers, M. L., Lengger, S. K., Bernasconi, S. M., Thibault, N., Schultz, B. P., Fernandez, A., Ullmann, C. V., McCormack, P., Bjerrum, C. J., Rasmussen, J. A., Hougård I. W., and Korte, C.: Cold spells in the Nordic Seas during the early Eocene Greenhouse, Nat. Commun., 11, 4713, https://doi.org/10.1038/s41467-020-18558-7, 2020.
Wang, Z., Wang, J., Suess, E., Wang, G., Chen, C., and Xiao, S.: Silicified glendonites in the Ediacaran Doushantuo Formation (South China) and their potential paleoclimatic implications, Geology, 45, 115–118, https://doi.org/10.1130/G38613.1, 2017.
Weaver, R., Roberts, A. P., Flecker, R., Macdonald, D. I. M., and Fot`yanova, L. M.: Geodynamic implications of paleomagnetic data from Tertiary sediments in Sakhalin, Russia (NW Pacific), J. Geophys. Res., 108, 2066, https://doi.org/10.1029/2001JB001226, 2003.
Williscroft, K., Grasby, S. E., Beauchamp, B., Little, C. T. C., Dewing, K., Birgel, D., Poulton, T., and Hryniewicz, K.: Extensive Early Cretaceous (Albian) methane seepage on Ellef Ringnes Island, Canadian High Arctic, Geol. Soc. Am. Bull., 129, 788–805, https://doi.org/10.1130/B31601.1, 2017.
Zabel, M. and Schulz, H. D.: Importance of submarine landslides for non-steady state conditions in pore water systems–lower Zaire (Congo) deep-sea fan, Mar. Geol., 176, 87–99, https://doi.org/10.1016/S0025-3227(01)00164-5, 2001.
Zharov, A.: South Sakhalin tectonics and geodynamics: A model for the Cretaceous-Paleogene accretion of the East Asian continental margin, Russ. J. Earth Sci., 7, ES5002, https://doi.org/10.2205/2005ES000190, 2005.
Zhou, X., Lu, Z., Rickaby, R. E. M., and Domack, E.: Ikaite Abundance Controlled by Porewater Phosphorus Level: Potential Links to Dust and Productivity, J. Geol., 123, 269–281, https://doi.org/10.1086/681918, 2015.
Zou, Z., Bertinetti, L., Habraken, W. J. E. M., and Fratzl, P.: Reentrant Phase Transformation from Crystalline Ikaite to Amorphous Calcium Carbonate, Cryst. Eng. Comm., 20, 2902–2906, https://doi.org/10.1039/C8CE00171E, 2018.