the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
BENFEP: a quantitative database of benthic foraminifera from surface sediments of the eastern Pacific
Paula Diz
Víctor González-Guitián
Rita González-Villanueva
Aida Ovejero
Iván Hernández-Almeida
Benthic foraminifera are important components of the ocean benthos and play a major role in ocean biogeochemistry and ecosystem functioning. Generating ecological baselines for ocean monitoring or biogeographical distributions requires a reference dataset of recent census data. Moreover, the information from their modern biogeography can be used to interpret past environmental changes on the seafloor. In this study, we provide the first comprehensive quantitative benthic foraminifera database from surface sediments of the eastern Pacific (BENFEP). Through the collation of archival quantitative data on species abundance and their homogenization according to the most recent taxonomic standards, we are able to provide a database with 3077 sediment samples, corresponding to 2509 georeferenced stations of wide geographical (60∘ N–54∘ S) and water depth (0–7280 m) coverage. The quantitative data include living, dead, and living plus dead assemblages obtained from 50 published and unpublished documents. As well as describing the data collection and subsequent harmonization steps, we provide summarized information on metadata, examples of species' distributions, potential applications of the database, and recommendations for data archiving and publication of benthic foraminiferal data. The database is enriched with meaningful metadata for accessible data management and exploration with R software and geographical information systems. The first version of the database (BENFEP_v1, Diz et al., 2022a, https://doi.org/10.1594/PANGAEA.947086) is provided in short and long format, and it will be upgraded with new entries and when changes are needed to accommodate taxonomic revisions.
- Article
(12980 KB) - Full-text XML
-
Supplement
(581 KB) - BibTeX
- EndNote
The eastern Pacific extends from the tidewater glaciers at Alaska to the fjords of Chile, encompassing a habitat that integrates eight large marine ecosystems (Sherman, 1991) covering 10.7×1011 m2 (Fig. 1). Tropical and subtropical latitudes harbour exceptional levels of pelagic and benthic biodiversity as well as the presence of endemic species at the macro- and microorganism levels (e.g. Davies et al., 2017; Gooday et al., 2021). Several areas of the eastern Pacific Ocean are at severe risk of species loss (Finnegan et al., 2015; Yasuhara et al., 2020; UNESCO, 2022), and some regions have consequently been categorized as marine protected areas (Enright et al., 2021).
The eastern Pacific is influenced by ocean–atmosphere natural climate variability modes at decadal–multi-decadal (e.g. El Niño–Southern Oscillation, the Pacific Decadal Oscillation, and the North Pacific Gyre Oscillation; Stuecker, 2018), millennial (Pisias et al., 2001), and glacial–interglacial (e.g. Walczak et al., 2020) timescales. These processes resulted in changes in temperature (Liu and Herbert, 2004), salinity (Praetorius et al., 2020), and productivity (Costa et al., 2017) in the surface ocean and oxygen concentrations in the bottom waters (Cannariato and Kennett, 1999). In a historical context, the increase in ocean temperatures and the expansion of the pre-existing extensive oxygen minimum zone (found at about 100–900 m water depth; Karstensen et al., 2008) are the major threats to the shallow and deep-water benthic ecosystems of the eastern Pacific (Sweetman et al., 2017; Breitburg et al., 2018; Yasuhara et al., 2019). These attributes make the eastern Pacific an area of interest for assessing the past, present, and future of the marine ecosystem status as well as its response to expected environmental changes (e.g. Calderon-Aguilera et al., 2022).
The Ocean Decade Implementation Plan (2021–2030) (https://www.oceandecade.org/, last access: August 2022), promoted by the United Nations, establishes several priority objectives for ocean sustainable development and conservation, which include a more profound understanding of benthic ocean ecosystem functioning and a better assessment of the vulnerability of coastal and deep-ocean areas to the ongoing impacts of anthropogenic activities and climate change. Attaining such targets might be challenged by the scarcity and unevenness of recent benthic organism census data that might function as suitable natural baselines (Yasuhara et al., 2012; Kidwell, 2015; Borja et al., 2020). Benthic foraminifera, microscopically sized and shelled organisms generally ranging from 63 to 1000 µm (Murray, 2006), are major components of the marine benthos. Those whose shells are composed of calcium carbonate have the potential to be preserved in the marine sediments, providing an ideal natural archive for recording past seafloor conditions.
Benthic foraminifera have been used for decades as past environmental indicators (Jorissen et al., 2007) and, more recently, in environmental monitoring (Alve et al., 2016; Jorissen et al., 2018). For example, in the eastern Pacific, benthic foraminifera have been used as proxies for changes in productivity (e.g. Patarroyo and Martinez, 2015; Diz et al., 2018; Tapia et al., 2021) and intermediate and deep-water oxygenation (e.g. Cannariato and Kennett, 1999; Tetard et al., 2017; Sharon et al., 2020). The proxy value of benthic foraminifera as palaeoenvironmental or biomonitoring tools could be hampered if the full scope of current biodiversity patterns, spatial distributions, and species–environment relations are not fully known or are grounded on a limited number of observations (e.g. Jorissen et al., 2007). A synthesis effort of recent benthic foraminiferal quantitative occurrences would definitively lead to a more complete picture of biogeographical distributions and relationships between environmental parameters and species composition, thereby rendering the interpretation of the fossil record more meaningful.
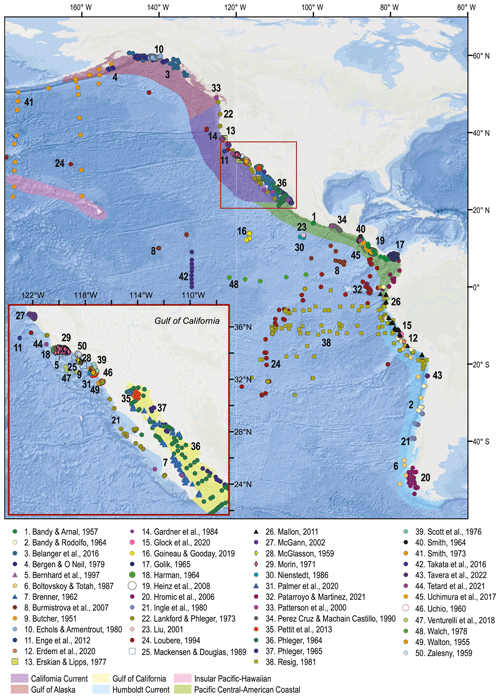
Figure 1Spatial distribution of the samples comprising the BENFEP_v1 database. The numbers refer to each author's dataset (see Table A1 for additional information). The shaded areas represent the large marine ecosystems of the eastern Pacific overlapping BENFEP_v1. The map was made using ArcGIS software (version 10.8.2). The global relief model integrates land topography and ocean bathymetry using information from Esri, Garmin, GEBCO, NOAA NGDC, and other contributions.
The data synthesis of marine microfossils from surface sediments has been a valuable resource among the palaeoceanographic community. They are generally used for constructing modern analogues to interpret the fossil record and, more recently, to evaluate the biodiversity response to ongoing climate change (e.g. Jonkers et al., 2019; Yasuhara et al., 2020). However, existing compilations of marine microfossils covering large ocean swathes mainly integrate census data on planktonic organisms dwelling in the first 100 m of the water column, such as planktic foraminifera (Siccha and Kucera, 2017), dinoflagellates (Marret et al., 2020), radiolarian (Boltovskoy et al., 2010; Hernández-Almeida et al., 2020), diatoms (Leblanc et al., 2012), or coccolithophores (Krumhardt et al., 2017). Public databases focused on the quantitative surface distribution of benthic microfossils are being developed for ostracods (e.g. Cronin et al., 2021; see also the review by Huang et al., 2022). Existing quantitative benthic foraminifera datasets from surface sediments including a relatively large number of stations (<300) are restricted to specific ocean sectors, size fractions, or test composition. Examples of these publicly available benthic foraminifera databases are those developed for the Norwegian continental shelf (Sejrup et al., 2004), which includes 298 stations and contains only calcareous foraminifera; the Indian Ocean (De and Gupta, 2010), with 131 core-top samples; or the central Arctic Ocean (Wollenburg and Kuhnt, 2000), with 90 stations. In the eastern Pacific, the science community has performed sporadic research efforts to attain an overview of the quantitative distributions of benthic fauna (e.g. Lankford and Phleger, 1973, n=102; Resig, 1981, n=121; and Loubere, 1994, n=66, where n indicates the number of samples with quantitative data). However, the large area to cover and the economic and time-related efforts required to sample a significant portion of the seafloor sediments of the entire eastern Pacific have prevented the construction of a large and consistent database of benthic fauna for this region.
In this paper, we present BENFEP, a quantitative database of benthic foraminifera from surface sediments of the eastern Pacific. The first version of the database (BENFEP_v1) contains a rich collection of metadata (e.g. research vessel, sampling devices, processing methods, etc.) and quantitative data (presented as percentages, counts, and densities) of harmonized benthic foraminiferal taxa obtained from more than 3000 samples of living, dead, and living plus dead assemblages gathered from published and unpublished studies. Here, we provide a complete description of the steps taken to build the database, its limitations, and the potential products for diverse stakeholders. BENFEP is structured to be analysed with data science tools and geographic information systems software.
2.1 BENFEP_v1 briefing
The first version of BENFEP (BENFEP_v1) integrates metadata and georeferenced quantitative data on benthic foraminifera species (living, dead, and living plus dead) from surface sediment samples collated from 50 published and unpublished documents released between 1951 and 2022. The number of samples supplied by each publication to the database varies among authors (see Table A1). The database includes samples ranging from 60∘ N to 54∘ S (Fig. 1) and localized from intertidal waters (0 m water depth) to the deepest curated sample at 7280 m water depth. BENFEP includes 2509 stations, 3077 samples, and 1091 foraminiferal taxa (including species-level and below-species-level designations) as well as 400 benthic foraminiferal identifications to the genus level.
2.2 Data source and selection protocols
The BENFEP_v1 database incorporates entries with georeferenced quantitative data on benthic foraminifera species from the eastern Pacific surface sediments. We consider data as quantitative when the species abundance in an assemblage is provided as the number of individuals (counts), the relative abundance (percent), or the density (number of individuals per volume unit). Primary sources of information for middle to late twentieth-century entries were the compilations by Culver and Buzas (1985, 1986, 1987), Ingle and Keller (1980), and the historical references by Finger (2013). For more recent publications, we used the search engines of Scopus, the Journal of Foraminiferal Research, JSTOR, and PANGAEA (accessed between early 2020 and March–December 2022), using the keywords “benthic foraminifera” along with geographic terms, such as “Eastern Pacific”, or specific geographical terms related to this region, such as “California”, “Chile”, “Santa Barbara”, “Alaska”, etc., as well as the authors' collaboration network. A total of 31 documents were published between 1929 and 2019 characterizing assemblages of living and dead assemblages of benthic foraminifera from surface sediments in the eastern Pacific that could not be incorporated in BENFEP_v1 because species assemblage data were provided in graphs, in terms of species presence, or as a range of abundances (e.g. common, rare, abundant). The geolocation of the samples and the authors of those publications can be accessed at https://doi.org/10.1594/PANGAEA.947114 (Diz et al., 2022b), and they are represented in Fig. B1.
A substantial number of entries used in BENFEP_v1 come from print-only publications, including unpublished theses accessed through universities and interlibrary loans (91 %). From these, only 7.6 % could be digitized, and the remaining (typewritten or handwritten tables) had to be converted to digital format manually (92.4 %). In those cases, entries were double-checked or, when necessary, tripled-checked to minimize errors and as a quality control. Moreover, BENFEP_v1 retains the original format in which census data were published, with percentage, counts, or densities representing 69 %, 30.7 %, and 0.3 % of the data respectively. It also includes any non-numerical data used by authors in their original publication to indicate the presence of a particular species or non-quantitative values of a particular species (e.g. “x”, “<1”).
2.3 Data geolocation
The samples integrated in BENFEP were georeferenced using the coordinates listed in the original publications. In Smith (1964) and Walton (1955), coordinates were not indicated in the original publication along with the benthic census data, and they had to be retrieved from another publication that used the same stations (Smith, 1963; Walton, 1954). For 30.3 % of samples, the location was only shown on maps. In those cases, the maps from the publications were digitized to raster format and georeferenced through ArcGIS software using geographic decimal degrees and the World Geodetic System of 1984 (WGS 84 – EPSG:4326). These rasters were then displayed with ArcGIS to extract the sample geolocation by manual digitizing. In cases where the resolution and precision of the map provided in the publication were clearly insufficient, the present coastline was retrieved using high-resolution satellite and aerial world imagery (World Imagery WMS server), and the samples' geolocations were obtained by combining both sources of data. It is worth mentioning that the coarse resolution of some hand-drawn maps, particularly those published in mid-twentieth-century surveys, might not be totally accurate.
All of the obtained geolocations were plotted as point features using high-resolution satellite and aerial world imagery as a base map to validate their position. In cases where the sample location resulted in an inland position, the data were cross-validated and checked; from these analyses, there were two possibilities: (i) typing errors in the original source or (ii) land reclamation activities in the area since the sample was collected. A few samples (11) were not georeferenced because the sample location was missing from maps (or lists provided by the authors) or the samples (2) are currently located inland.
2.4 Taxonomic harmonization
The datasets contributing to BENFEP_v1 come from multiple sources published over the last 70 years; therefore, taxonomic inconsistencies between authors are expected. Aiming to harmonize the spectra of genera and species from the original sources, we standardized the original taxonomy using the currently valid taxonomic assignments of the World Foraminifera Database (Hayward et al., 2022), a part of the World Register of Marine Species (WoRMS). In order to find the valid species name, we searched each author's original species assignment in the WoRMS research engine. This procedure enabled us to identify whether the original species name was accepted (valid species) or if it was a synonym of a valid species or a taxa corresponding to a variety or a subspecies. When the original species name was not currently in use, it was substituted by the valid species, subspecies, or variety name. Species names annotated with “cf.” or “aff.” were not considered to be separate species. Some taxa included in BENFEP_v1 are considered “fossil only” by WoRMS; nevertheless, we retained those in the database. There are several reasons to explain the occurrence of a species categorized as “fossil only” in a sample: it represents a true displaced fossil species from ancient sediments (reworking), it is a mistaken identification, or it is an extant species inaccurately categorized as a fossil by WoRMS. As it is not clear which of these circumstances applies in each case, we decided to retain the species to prevent information loss in the case of a future re-evaluation of the “fossil range” by WoRMS. The species identified to the genus level with only one species by one author (e.g. Genus A sp.) were assigned to the column name designed by the genera followed by “spp.” (e.g. Genus A spp.). However, if an author indicated two or more “sp.” species for the same genus (e.g. Genus B sp1, Genus B sp2), a column name with the undetermined species followed by the author's name is used (e.g. Genus B sp1Golik, where “Golik” refers to the dataset of Golik, 1965). The columns named “Indeterminate calcareous” and “Indeterminate agglutinated” included individuals not identified at the genus nor species level in the original publication and included in more general categories such as “other calcareous”, “miliolids”, “lagenids”, or “other agglutinated”. When authors did not provide information about the test nature (e.g. agglutinated or calcareous), census data on the non-identified forms were placed in the “Indeterminate unknown” column. The WoRMS search engine was last accessed on 8 December 2022 using the “worrms” package (Chamberlain, 2020) through R version 4.2.1 (R Core Team, 2022) to obtain the updated scientific names, authorities, AphiaID, rank, and species “fossil range” (renamed as “occurrence” in this study). The taxonomic information retrieved from WoRMS, the authors' original assignations, and the authors' original specific remarks on the harmonization procedure are included in the Supplement Files 1, 2, and 3 respectively. Extended explanations about some species, in particular those referred to as “potentially fossil” by the original authors, are included in File 4 in the Supplement. Formal discussions of the taxonomic concepts used by the authors of the publications and by WoRMS are outside the scope of this study.
2.5 Structure of the database
The BENFEP_v1 database is provided in short and long format to reach a high spectrum of final users. The short format (BENFEP_v1_short) consists of 3077 rows and 1565 columns. Each row contains information on one surface sample distributed in metadata (columns 1–23 and columns 1556–1565), aiming to provide all of the necessary information for users to assess the quality of the faunal dataset and manage the data at their own convenience. The metadata for each sample were collated from the original source and include information about the publication, the name of the research vessel used to collect the sample, the sampling year, details regarding different sampling methodologies such as sampling devices and sampling interval (in centimetres at the seabed), the format in which the quantitative data were originally published (percent, counts, density), the type of assemblage (living, dead, or living plus dead), the size fraction in which foraminifera were studied, picking and staining protocols to identify living foraminifera, the geolocation (latitude and longitude), and the water depth of the surface sediment sample. We also included where we obtained the data from (provided by authors, obtained from machine or manual digitization, or retrieved from repositories), the DOI of the dataset when hosted in an open-access repository, and the source of the geographic coordinates (obtained from tables in the publication or digitized maps) as metadata. Additionally, in columns 1556–1565, we coded whether the number of counted individuals in each sample was equal to or higher than 100, 200, or 300 individuals. Meaningful annotations regarding the sample entry were spared in seven columns dedicated to the meaning of non-numerical data, comments about some species, assemblage characteristics, the volume of the sample (when data are provided in density), the size fraction, the sample geolocation, and others. Benthic foraminifera species quantitative data, comprising one taxon per column, are indicated in columns 24 to 1554. The species, varieties, and subspecies names are identified in full in one column (e.g. genus and species or genus, species, and variety or subspecies). A column representing the sum of species abundance per sample (“total” column) was added at the end of the species quantitative data. Users should check the “format” column for indications as to whether the value in the column represents the sum of percentages, counts, or densities. An empty cell in any column indicates that there is no information available. The users of the short format are referred to File 1 in the Supplement for comprehensive taxonomic information on each taxa and to File 2 in the Supplement for the original authors' taxonomic concepts.
The long format of BENFEP_v1 (BENFEP_v1_long) contains 33 columns reflecting the metadata described above for BENFEP_v1_short and three columns describing the harmonized foraminiferal designation (“entity”), each species quantitative data (“abundance”), and the total abundance in the sample (“total”, see the “format” column). This information is followed by the taxonomic information extracted from WoRMS (“valid_authority”, “status”, “rank”, “AphiaID”, “kingdom”, “phylum”, “class”, “order”, “family”, “genus”, “occurrence”) and each author's taxonomic concept (“authors_taxo”). Tables C1 and C2 detail the meaning of each column and the column codes of the respective BENFEP_v1_short and BENFEP_v1_long databases. The database in its two versions is presented in text format and can be managed with virtually any software.
3.1 Sample distribution
The sample distribution in BENFEP_v1 is dictated by the availability of, and access to, benthic foraminifera quantitative datasets. The geographic range of samples varies between 60∘ N and 54∘ S and from 70 to 179∘ W. The largest density of quantitative data occurs between 40 and 30∘ N, followed by groups of stations centred at 60∘ N and between 10 and 17∘ N (Fig. 1; Video Supplement). There are some spatial gaps in benthic foraminifera census data, such as the regions between 17 and 21∘ N and several narrow latitudinal intervals in the Southern Hemisphere (40–45, 36–39, 33–35, 29–31∘ S). The water depths range from tidal (0 m) to 7280 m, but 50 % of stations were collected between water depths of 40 and 550 m (Fig. 2). From Figs. 1 and 2, it remains clear that eastern Pacific Ocean areas deeper than 3000 m (i.e. lower abyssal zones, following van Morkhoven et al., 1986) are noticeably understudied and that far more studies are needed in these regions to obtain a full overview of benthic foraminiferal distributional patterns. Indeed, the highest number of samples in lower abyssal environments (deeper than 3000 m; Fig. 2) is from the southern Pacific, and they come from expeditions carried out during the 1960s and 1970s (Bandy and Rodolfo, 1964; Resig, 1981).
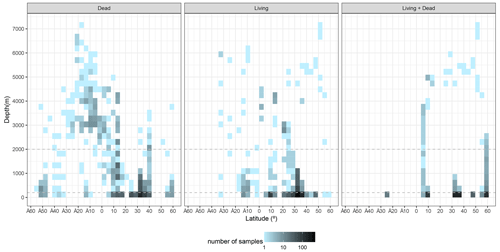
Figure 2Distribution of samples with water depth and latitude. Horizontal dashed lines separate the neritic (0–200 m), the bathyal (200–2000 m), and the abyssal (>2000 m) zones following the bathymetric divisions of van Morkhoven et al. (1986). The graphs were elaborated with the “tidyverse” package (Wickham et al., 2019) using R version 4.2.1 (R Core Team, 2022).
3.2 Research vessels, sampling devices, and sampling intervals
Research expeditions were carried out aboard different research vessels; VELERO IV, Spencer F. Baird, McArthur, Yaquina, Golden West, Atlantics II, Puritan, Horizon, and Meteor are some of the 35 cited research vessels (information taken from “rv_1” and “rv_2”; see Appendix C). Alternatively, some samples were provided by miscellaneous collections from the Scripps Institution of Oceanography and Allan Hancock Foundation.
Samples were collected using a variety of devices (at least 18 different samplers; Tables C1 and C2), but most of samples were taken using a gravity corer (20.5 %) as well as Hayward orange peel grabs, box corers, Phleger corers, and miscellaneous tools (mostly in shallow water depths; percentages of around 8 %–15 % each; Fig. 3). The most common sediment sampling interval below the seafloor is 0–1 cm (41.4 %), where benthic foraminifera are distributed between dead (9.9 %), living (24 %), and living plus dead (7.6 %) assemblages. Slightly deeper sampling intervals (e.g. 0–2, 0–3, and 0–5 cm; Fig. 3) represent 38.4 % of the samples in the database (Fig. 3). A total of 20.2 % of the samples are classified as “surface samples”, representing the authors' generic assignations to the uppermost centimetre of the sediment (e.g. “surface”, “core-top”).
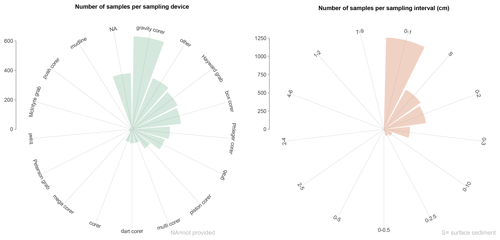
Figure 3Sampling devices and sampling intervals in BENFEP_v1. The distribution of sampling devices is calculated using the “dev_1” column (see Tables C1 and C2 for more information). The graphs were elaborated with the “tidyverse” package (Wickham et al., 2019) using R version 4.2.1 (R Core Team, 2022).
3.3 Benthic foraminiferal assemblages
The BENFEP_v1 database reports data on living (40.1 %), dead (33.6 %), and living plus dead (26.3 %) benthic foraminifera. Rose bengal staining (Walton, 1952) is the only method used by authors to distinguish dead (unstained) from living (stained) foraminifera at the time of sampling. Living plus dead refers to an assemblage where living (stained) and dead (unstained) are counted together in the same sample. The stain is mixed with different solvents, with the most commonly used being formaldehyde (54.8 %), followed by alcohol (19.7 %) and “others”, the latter of which includes seawater and distilled water (25.5 %). Samples were mainly dry picked after flotation (54 %) with a dense liquid (mostly Cl4C), which was common practice between 1951 and 1980.
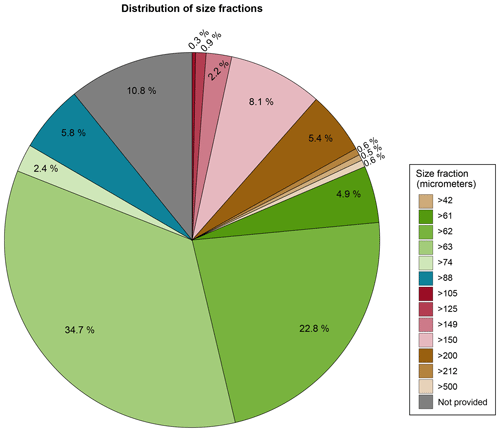
Most of the benthic foraminiferal assemblages were analysed in the smallest size fractions commonly used in benthic foraminiferal studies. For example, 65.4 % of the samples were analysed using 42, 61, 62, 63, and 74 µm as the lower end of the size fraction (e.g. assemblages were studied in the respective >42, >61, >62, >63, and >74 µm size fraction). A total of 6.1 % were analysed using 88 and 105 µm as the lower end of the size fraction, and 17.7 % were analysed using 125, 149, 150, 200, 212, and 500 µm as the lower end of the size fraction. The size fraction used for foraminiferal analysis was not reported in 10.8 % of the publications (Table A1 and Fig. 4), corresponding to four entries: Phleger (1965), Lankford and Phleger (1973), Bergen and O'Neil (1979), and the historical data reported by McGann (2002).
3.4 Benthic foraminiferal species
The BENFEP_v1 dataset includes a total of 1091 valid taxa (1073 species, 14 varieties, 4 subspecies) as well as two taxa of uncertain status (Serpula lobata and Ammonia avalonensis) corresponding to 335 foraminiferal genera belonging to the classes Globothalamea (64 %), Tubothalamea (11.3 %), Nodosariata (19.6 %), and Monothalamea (4.8 %). In addition to the accepted taxonomic entities, the database contains 400 benthic foraminifera individuals identified to the genus level (i.e. “spps”). The genus with the largest number of valid species (excluding subspecies and varieties) is Bolivina (46), followed – in decreasing order – by Quinqueloculina, Uvigerina, Reophax, Fissurina, Lagena, and Bulimina (22–32 species; Fig. 5, see also File 1 in the Supplement).
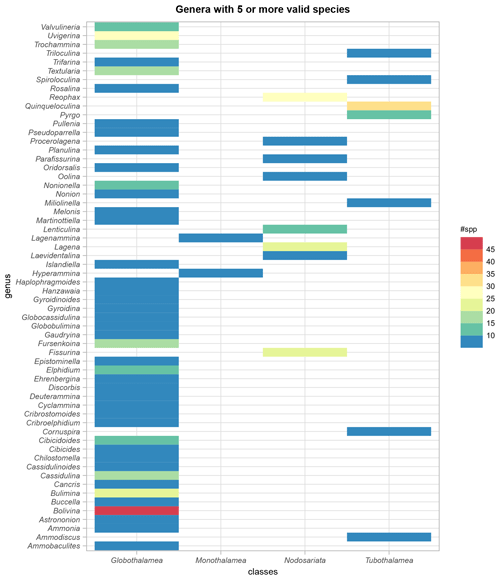
Figure 5Number of valid species per foraminifera genus and their distribution among the classes, as indicated by WoRMS (Hayward et al., 2022). Only genera with five or more species are represented in the figure. The graph was elaborated with the “tidyverse” package (Wickham et al., 2019) using R version 4.2.1 (R Core Team, 2022).
The BENFEP_v1 database contains 292 valid species (excluding varieties and subspecies) that can be considered rare, with a mean relative contribution lower than 1 % (as per the definition of “rare” from Murray, 2013) (calculation based on samples with counts above 100 individuals analysed in the >61, >62, >63, >74, and >88 µm size fractions). Furthermore, the highest number of taxa (90) is found at a station studying dead individuals located in the southern Pacific at 1800 m water depth (Fig. 6d; Ingle et al., 1980). BENFEP_v1 integrates quantitative data across a variety of marine environments; thus, the relative abundance of particular species varies geographically and with water depth (Fig. 6a, b, e). For example, Textularia mariae, Elphidium excavatum spp. clavatum, and Globocassidulina crassa are frequent in the neritic zone, whereas Nodulina dentaliniformis and Nuttallides umbonifer characterize the abyssal zones (Fig. 6e).
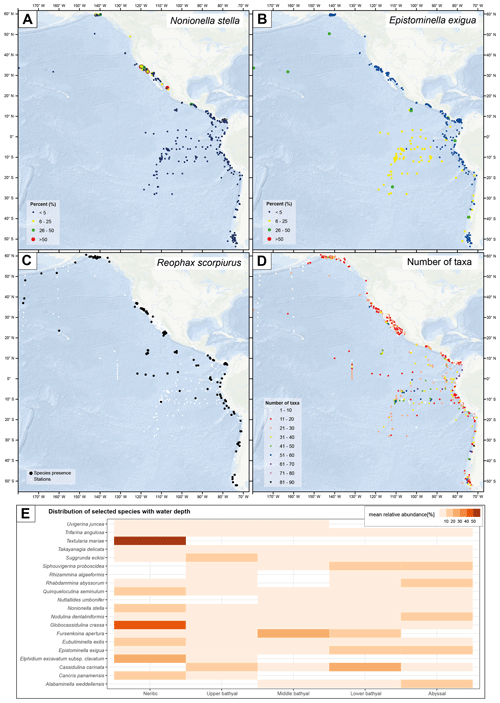
Figure 6Geospatial representation of selected species' relative abundance (a, b) and presence (c), total number of taxa (d), and selected species' mean relative abundance with water depth (e) in BENFEP_v1. Water depth ranges in panel (e) are as follows: neritic (0–200 m), upper bathyal (200–600 m), middle bathyal (600–1000 m), lower bathyal (1000–2000 m), and abyssal (>2000 m). The relative abundance of species in panels (a), (b), and (e) are calculated from a percentage file that integrates samples with counts of more than 100 individuals in the >61, >62, >63, >74, and >88 µm fractions. The calculations of species' presence (c) and total number of taxa (d) are calculated by integrating the information provided by non-numerical data. The maps shown in panels (a) to (d) were made using ArcGIS software version 10.8.2. The global relief model integrates land topography and ocean bathymetry using information from Esri, Garmin, GEBCO, NOAA NGDC, and other contributions. The graph in panel (e) was elaborated with the “tidyverse” package (Wickham et al., 2019) using R version 4.2.1 (R Core Team, 2022).
3.5 Potential applications of BENFEP
The high number of stations with benthic foraminifera quantitative data collated from surface sediments of the eastern Pacific as well as the metadata provided make BENFEP_v1 a reference database for a specialized community working on present and past benthic foraminiferal distributions. The database has the potential to be integrated with other databases hosting taxonomic, abundance, or biogeographic information on other microfossils, thereby serving as a source of ecological information (e.g. biodiversity, ecosystem functioning) for shallow and deep-sea monitoring, management, and conservation (Danovaro et al., 2020). Figure 6 displays some of the potential applications of BENFEP, ranging from the relationship between species and a particular environmental variable (i.e. water depth; Fig. 6e) – which can be extended to another, externally accessed environmental variable, the geographic distribution of the relative abundance of species (Fig. 6a, b) – to species presence (Fig. 6c) and the number of taxa (Fig. 6d).
3.6 Limitations of the database
3.6.1 Taxonomic concepts
The species-level taxonomy of benthic foraminifera is mainly based on morphological traits, whose identification criteria might differ among authors, particularly if we consider the time elapsed between some publications. This could represent a limitation that is shared among global or regional databases curating published data from other modern marine microfossil groups (Leblanc et al., 2012; Siccha and Kucera, 2017; Hernández-Almeida et al., 2020). However, the effect of diversified taxonomic concepts might be augmented in benthic foraminifera, whose modern taxa (2400 living species; Murray, 2007) outnumber other marine microorganism groups with fossilizing potential, such as planktonic foraminifera (n=50 living species; Brummer and Kucera, 2022), coccolithophores (n=200 extant species; Young et al., 2003), or radiolarian, with at least 900 species (Biard, 2022). Despite the effort to harmonize the taxonomy, it is likely that incorporating data from different authors with diverse taxonomic concepts (e.g. there are 499 species identified by a single author) and potential misidentifications (e.g. see File 4 in the Supplement) could have artificially biased the number of species.
3.6.2 Data originally sourced in percentage
The data provided in percentage sometimes do not add to 100 %. There are several explanations for this. Firstly, the presence of symbols (such as “x”, “<0.1”) or incomplete assemblage descriptions (e.g. datasets including only species beyond a particular threshold in their relative abundance) necessarily preclude that the sum of the relative contribution of species reaches 100 %. We refer users to the “remark_1” and “remark_3” sections of the database for additional information about the assemblage characteristics (see Tables C1 and C2). Secondly, the rounding of decimals to entire numbers in the original sources might have led to percentages lower or higher than 100 %. A few samples from Butcher (1951) contain well above 100 %. We hypothesize that they are probably the result of typing errors in the original sources. In any case, we decide to retain quantitative data in their unabridged form because there are potential applications of the database insensitive to percentages, such as species presence.
3.6.3 Non-numerical data
There are 18 datasets that include non-numerical data (“x”, “<1”) in their records (see “remark_1”). Those data might interfere with the calculation of the relative abundances and some diversity indexes (e.g. Shannon–Weaver). However, they provide useful information on species presence, and therefore they are potentially useful for biogeography and calculations of species richness. General suggestions on how to manage non-numerical data in R can be found in File 5 in the Supplement.
3.6.4 The representativeness of the surface sediment assemblages as recent analogues
One of the purported applications of BENFEP_v1 is to provide a quantitative estimate of recent benthic foraminiferal assemblages that could later be used in palaeoenvironmental interpretations (e.g. Fig. 6). The database integrates quantitative data obtained from oceanic regions with different depositional environments, sedimentation rates, carbonate preservations, and types of assemblages, collected over different sampling years and using an array of sampling devices that might result in diversion from recent conditions. For example, dead benthic foraminifera obtained from surface sediments might not be representative of the surface if the sampling device fails to recover the sediment–water interface or if the sedimentation rates are very low. A total of 36 % of the surface sediment samples were retrieved using different types of coring devices (gravity, piston, dart, and Phleger corer; calculations using “dev_1”), which are sampling techniques that can cause perturbation or mis-sampling of the surface sediment (Weaver and Schultheiss, 1990). As the studies included in our database did not date the surface sediment (except for Palmer et al., 2020), we cannot discard that some samples correspond to pre-Holocene conditions. The most comprehensive compilation of sedimentation rates from core-top samples is from the equatorial Pacific and shows highly variable values, ranging from 0.8 to 14.2 cm kyr−1 (Mekik and Anderson, 2018), meaning that surface sediment samples in this region correspond to recent conditions (assuming that no perturbation occurred during sampling). Reworking, downslope transport, and carbonate preservation might be other factors influencing the composition of the assemblages obtained from the surface sediments. The presence of “potentially fossil” species reworked from ancient outcrops (see “remark_2” and File 4 in the Supplement) is included in the datasets of Bandy and Arnal (1957), Echols and Armentrout (1980), Ingle et al. (1980), and Zalesny (1959). However, they represent less than 5 % of the assemblage. The contribution of displaced specimens from shallower locations is also low, as indicated by Bandy and Arnal (1957), Ingle et al. (1980), Harman (1964), Pettit et al. (2013a), Uchimura et al. (2017), and Zalesny (1959). Finally, Pettit et al. (2013a), in the Gulf of California, and Boltovskoy and Totah (1987) and Resig (1981), off South America in samples below the carbonate compensation depth, are the only authors mentioning the poor preservation of calcareous benthic foraminifera.
BENFEP_v1 includes information on living, dead, and living plus dead assemblages whose suitability for building recent analogues is under discussion among the scientific community. The use of rose bengal as “vital” staining could be controversial because attached bacteria/algae or the decaying protoplasm of dead individuals might stain, resembling the staining of the protoplasm of a “true” living individual (see review in Schönfeld, 2012). However, it is still the most widely used method to distinguish “living” (stained) from “dead” (unstained) foraminifera, and it is considered reliable if used cautiously. It might be argued that only living foraminifera should be used to consider baseline studies (Schönfeld, 2012). However, it might also be considered that living assemblages represent a “snapshot” of the foraminifera living at the specific time of sampling and do not hold the time-averaged representativeness of the dead assemblages (Murray, 2000). Regarding all of these potential concerns, we have incorporated a rich collection of metadata in BENFEP_v1 that can be used by the final users to evaluate data quality and to tailor the final output to their specific criteria.
Data sharing in easily accessible formats and public repositories should be the core of the commitment of scientists, universities, and research institutions to open science. Data reuse is not only precluded by lack of data sharing but also by incomplete or lacking metadata, taxonomic information, etc., which are essential to provide the single user or the synthesizer with the information required to evaluate the quality of data. In the process of building this database, we have found several issues that we raised as recommendations, aiming to encourage best practices in data reporting:
-
Data sharing. Publishers should commit to FAIR (findability, accessibility, interoperability, and reusability) data practices (Wilkinson et al., 2016), and authors must share their published data in a readily accessible format and in public repositories to avoid the irreversible loss of valuable quantitative data. An important disadvantage of machine and manual digitalization is that both are time-consuming and might result in typing errors.
-
Raw data. Ensuring the reproducibility, quality checking, and further use of data requires raw data (i.e. species counts and total counts per each sample). It has been common practice to provide quantitative data on relative abundance with generic information about the number of individuals counted by sample. As mentioned before, this format is prone to error and hinders, at least, data reuse for some diversity calculations (e.g. rarefaction).
-
Metadata. Detailed information should be provided regarding each station's sampling device, sampling interval, geographic coordinates, picking and staining protocols, research vessel, sampling year, etc. The description of samples' metadata using unspecific generalizations should be avoided.
-
Taxonomy. Full taxonomic references of all species should be provided. Taxonomic information and supporting images are crucial elements for reliable taxonomic harmonization and data reusability.
The BENFEP_v1 database is available through PANGAEA Data Publisher (Diz et al., 2022a, https://doi.org/10.1594/PANGAEA.947086). This database is conceived as a springboard to store future quantitative data on benthic foraminifera in the eastern Pacific and make them available to the scientific community. It will be open for any new quantitative data entry and, thus, welcomes any new data published or provided by any contributor. The authors will update the database once a considerable number of new entries need to be incorporated or changes are required to update taxonomic categories to an existing version. New versions of BENFEP will be submitted and curated using PANGAEA. Collaborations with individual researchers and institutions are welcomed, especially regarding potential expansion to other ocean basins. Complementary information to BENFEP_v1 can be found in Diz et al. (2022b) (https://doi.org/10.1594/PANGAEA.947114) and Diz et al. (2022c) (https://doi.org/10.5281/zenodo.7472278).
We present the BENFEP database, the largest open-access database of quantitative data on benthic foraminifera from surface sediments compiled to date. BENFEP_v1 contains harmonized census counts of 1091 foraminiferal taxa (including species- and below-species-level designations) of living, dead, and living plus dead benthic foraminifera from 3077 sediment samples, corresponding to 2509 stations in the eastern Pacific. It also contains a rich collection of metadata gathered from 50 documental sources spanning the last 70 years. The prospective of BENFEP_v1 is to function as an active repository for new entries and a reference database for palaeoenvironmental reconstructions as well as biogeography and biomonitoring studies. The database is coded in a friendly manner and can, thus, be accessed using different software, with the aim of servicing a broad spectrum of users and allowing them to tailor the database to their needs.
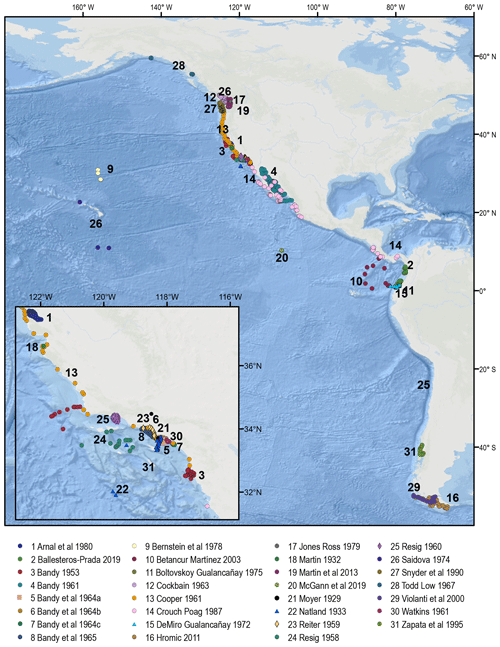
Figure B1Spatial distribution of samples in the eastern Pacific from studies that do not provide quantitative assemblage data. The numbers refer to each author's dataset. The sample geolocation and metadata can be found at https://doi.org/10.1594/PANGAEA.947114 (Diz et al., 2022b). The procedures for stations' georeferencing and column coding are outlined in Sect. 2.3 and 2.5 respectively. The map was made using ArcGIS software version 10.8.2. The global relief model integrates land topography and ocean bathymetry using information from Esri, Garmin, GEBCO, NOAA NGDC, and other contributions.
Table C1Explanatory notes on the column names and column codes of BENFEP_v1_short.
An empty cell in any column indicates that there is no information available.
An accumulative timeline heat map showing the geographic distribution of samples' density in BENFEP_v1. The type of assemblage (dead, living, and living plus dead) is identified using black, red, and green filled circles respectively. The global relief model integrates land topography and ocean bathymetry from Esri, Garmin, GEBCO, NOAA NGDC, and other contributions. The slides for the video were made using QGIS software, and the video assembly was done with Adobe Premiere Pro software. The video supplement can be accessed at https://doi.org/10.5281/zenodo.7472278 (Diz et al., 2022c).
The supplement contains five files: File 1 indicates the systematics of benthic foraminiferal species listed in BENFEP_v1 following the concepts of the World Foraminifera Database (Hayward et al., 2022); File 2 lists the original authors' species designations for the species harmonized in BENFEP_v1 and indicated in File 1; File 3 contains specific remarks on the harmonization procedure; File 4 provides extended explanations about some species; and File 5 provides general suggestions on how to manage BENFEP_v1_short in R. The supplement related to this article is available online at: https://doi.org/10.5194/essd-15-697-2023-supplement.
IHA conceptualized the study. PD was responsible for metadata collation, benthic foraminifera curation, and species harmonization. PD and VGG cross-validated entries of manually digitized data carried out by VGG. RGV and AO were responsible for georeferencing stations and geographic data visualizations. PD was involved in organizing quantitative data for figures and statistics. All authors contributed to the design of the database structure and actively participated in organizing, writing, and editing the manuscript at its various stages.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors would like to acknowledge the efforts of the personnel of the Interlibrary Loan services of the Universidade de Vigo and ETH Zürich, who provided us with access to legacy documents. We also extend our acknowledgements to the librarians of Florida State University, University of California Los Angeles, University of California San Diego, University of Southern California, Stanford University, and Martin Luther King University, who provided access to parts of unpublished PhD and master's theses.
Victor González Guitián received funding from the Xunta de Galicia (grant no. GRC-ED431C 2017/55).
This paper was edited by Giuseppe M. R. Manzella and reviewed by Lukas Jonkers, Ellen Thomas, and two anonymous referees.
Alve, E., Korsun, S., Schönfeld, J., Dijkstra, N., Golikova, E., Hess, S., Husum, K., and Panieri, G.: Foram-AMBI: A sensitivity index based on benthic foraminiferal faunas from North-East Atlantic and Arctic fjords, continental shelves and slopes, Mar. Micropaleontol., 122, 1–12, https://doi.org/10.1016/j.marmicro.2015.11.001, 2016.
Arnal, R. E., Quinterno, P. J., Conomos, T. J., and Gram, R.: Trends in the distribution of recent foraminifera in San Francisco Bay, Cushman Foundation Special Publication, 19, 17–39, ISBN 9781970168129, 1980.
Ballesteros-Prada, A.: Modern Benthic Foraminifera “Phylum Foraminifera (D'Orbigny 1826)” of the Panama Bight: A Census Report Based on Thanatocoenoses from the Continental Slope, in: Advances in South American Micropaleontology Selected Papers of the 11th Argentine Paleontological Congress, edited by: Cusminsky, G. C., Bernasconi, E., and Concheyro, G. A., Springer Earth System Sciences, Springer Nature Switzerland AG, 175–213, https://doi.org/10.1007/978-3-030-02119-1_9, 2019.
Bandy, O. L.: Ecology and Paleoecology of Some California Foraminifera. Part I. The Frequency Distribution of Recent Foraminifera off California, J. Paleontol., 27, 161–182, http://www.jstor.org/stable/1300051 (last access: March 2022), 1953.
Bandy, O. L.: Distribution of Foraminifera, Radiolaria and Diatoms in Sediments of the Gulf of California, Micropaleontology, 7, 1–26, https://doi.org/10.2307/1484140, 1961.
Bandy, O. L. and Arnal, R. E.: Distribution of Recent Foraminifera Off West Coast of Central America, AAPG Bull., 41, 2037–2053, https://doi.org/10.1306/0BDA5957-16BD-11D7-8645000102C1865D, 1957.
Bandy, O. L. and Rodolfo, K. S.: Distribution of foraminifera and sediments, Peru-Chile trench area, Deep Sea Research and Oceanographic Abstracts, 11, 817–837, https://doi.org/10.1016/0011-7471(64)90951-9, 1964.
Bandy, O. L., Ingle Jr., J. C., and Resig, J. M.: Facies trends, San Pedro Bay, California, Geol. Soc. Am. Bull., 75, 403–424, https://doi.org/10.1130/0016-7606(1964)75[403:FTSPBC]2.0.CO;2, 1964a.
Bandy, O. L., Ingle Jr., J. C., and Resig, J. M.: Foraminifera, Los Angeles County outfall area, California, Limnol. Oceanogr., 9, 124–137, https://doi.org/10.4319/lo.1964.9.1.0124, 1964b.
Bandy, O. L., Ingle Jr., J. C., and Resig, J. M.: Foraminiferal trends, Laguna Beach outfall area, California, Limnol. Oceanogr., 9, 112–123, https://doi.org/10.4319/lo.1964.9.1.0112, 1964c.
Bandy, O. L., Ingle Jr., J. C., and Resig, J. M.: Foraminiferal trends, Hyperion oufall, California, Limnol. Oceanogr., 10, 314–332, https://doi.org/10.4319/lo.1965.10.3.0314, 1965.
Belanger, C. L., Orhun, O. G., and Schiller, C. M.: Benthic foraminiferal faunas reveal transport dynamics and no-analog environments on a glaciated margin (Gulf of Alaska), Palaeogeogr. Palaeocl., 454, 54–64, https://doi.org/10.1016/j.palaeo.2016.04.032, 2016.
Bergen, F. W. and O'Neil, P.: Distribution of Holocene Foraminifera in the Gulf of Alaska, J. Paleontol., 53, 1267–1292, http://www.jstor.org/stable/1304134 (last access: March 2022), 1979.
Bernhard, J. M., Sen Gupta, B. K., and Borne, P. F.: Benthic foraminiferal proxy to estimate dysoxic bottom-water oxygen concentrations; Santa Barbara Basin, U. S. Pacific continental margin, J. Foramin. Res., 27, 301–310, https://doi.org/10.2113/gsjfr.27.4.301, 1997.
Bernstein, B. B., Hessler, R. R., Smith, R., and Jumars, P. A.: Spatial dispersion of benthic Foraminifera in the abyssal central North Pacific, Limnol. Oceanogr., 23, 401–416, https://doi.org/10.4319/lo.1978.23.3.0401, 1978.
Betancur, M. J. and Martínez, I.: Recent benthonic foraminifera in deep-sea sediments of the Panama basin (Colombian Pacific), as indicators of productivity and oxygenation, Boletin de Investigaciones Marinas y Costeras, 32, 93–123, 2003.
Biard, T.: Diversity and ecology of Radiolaria in modern oceans, Environ. Microbiol., 24, 2179–2200, https://doi.org/10.1111/1462-2920.16004, 2022.
Boltovskoy, D., Kling, S. A., Takahashi, K., and Bjørklund, K.: World Atlas of Distribution of Recent Polycystina (Radiolaria), Palaeontol. Electron., 13, 1–229, 2010.
Boltovskoy, E. and Gualancañay, E.: Foraminiferos bentonicos actuales de Ecuador. 1. Provincia Esmeraldas, Instituto Oceanografico de la Armada Guayaquil-Ecuador, 1975, 56 pp., 1975.
Boltovskoy, E. and Totah, V. I.: Relación entre masas de agua y foraminiferos bentónicos en el Pacífico sudoriental, PHYSIS, Secc. A, 45, 37–46, 1987.
Borja, A., Andersen, J. H., Arvanitidis, C. D., Basset, A., Buhl-Mortensen, L., Carvalho, S., Dafforn, K. A., Devlin, M. J., Escobar-Briones, E. G., Grenz, C., Harder, T., Katsanevakis, S., Liu, D., Metaxas, A., Morán, X. A. G., Newton, A., Piroddi, C., Pochon, X., Queirós, A. M., Snelgrove, P. V. R., Solidoro, C., St. John, M. A., and Teixeira, H.: Past and Future Grand Challenges in Marine Ecosystem Ecology, Frontiers in Marine Science, 7, 362, https://doi.org/10.3389/fmars.2020.00362, 2020.
Breitburg, D., Levin, L. A., Oschlies, A., Grégoire, M., Chavez, F. P., Conley, D. J., Garçon, V., Gilbert, D., Gutiérrez, D., Isensee, K., Jacinto, G. S., Limburg, K. E., Montes, I., Naqvi, S. W. A., Pitcher, G. C., Rabalais, N. N., Roman, M. R., Rose, K. A., Seibel, B. A., Telszewski, M., Yasuhara, M., and Zhang, J.: Declining oxygen in the global ocean and coastal waters, Science, 359, eaam7240, https://doi.org/10.1126/science.aam7240, 2018.
Brenner, G. J.: Results of the Puritan-American Museum of Natural History Expedition to Western Mexico. 14, A zoogeographic analysis of some shallow-water Foraminifera in the Gulf of California, B. Am. Mus. Nat. Hist., 123, 5, 1962.
Brummer, G.-J. A. and Kučera, M.: Taxonomic review of living planktonic foraminifera, J. Micropalaeontol., 41, 29–74, https://doi.org/10.5194/jm-41-29-2022, 2022.
Burmistrova, I. I., Khusid, T. A., Belyaeva, N. V., and Chekhovskaya, M. P.: Agglutinated abyssal foraminifers of the equatorial pacific, Oceanology, 47, 824–832, https://doi.org/10.1134/S0001437007060070, 2007a.
Burmistrova, I. I., Khusid, T. A., Belyaeva, N. V. and Chekhovskaya, M. P.: (Table 2) Species composition of agglutinated foraminifers from the abyssal zone of the Pacific Ocean, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.726917, 2007b.
Butcher, W. S.: Part II Foraminifera, Coronado Bank and Vicinity, Calif., University of California, Los Angeles, 58 pp., https://bit.ly/3HJrEKk (last access: March 2022), 1951.
Calderon-Aguilera, L. E., Reyes-Bonilla, H., Morzaria-Luna, H. N., Perusquía-Ardón, J. C., Olán-González, M., and Méndez-Martínez, M. F.: Trophic architecture as a predictor of ecosystem resilience and resistance in the eastern Pacific, Prog. Oceanogr., 209, 102922, https://doi.org/10.1016/j.pocean.2022.102922, 2022.
Cannariato, K. G. and Kennett, J. P.: Climatically related millennial-scale fluctuations in strength of California margin oxygen-minimum zone during the past 60 k.y., Geology, 27, 975–978, https://doi.org/10.1130/0091-7613(1999)027<0975:crmsfi>2.3.co;2, 1999.
Chamberlain, S.: worrms: World Register of Marine Species (WoRMS) Client, R package version 0.4.2, https://CRAN.R-project.org/package=worrms (last access: 8 December 2022), 2020
Cockbain, A. E.: Distribution of foraminifera in Juan de Fuca and Georgia straits, British Columbia, Canada, Contributions from the Cushman Foundation for Foraminiferal Research, 14, 37–57, 1963.
Cooper, W. C.: Intertidal foraminifera of the California and Oregon Coast, Contributions from the Cushman Foundation for Foraminiferal Research, 12, 47–63, 1961.
Costa, K. M., Jacobel, A. W., McManus, J. F., Anderson, R. F., Winckler, G., and Thiagarajan, N.: Productivity patterns in the equatorial Pacific over the last 30 000 years, Global Biogeochem. Cy., 31, 850–865, https://doi.org/10.1002/2016GB005579, 2017.
Cronin, T. M., Gemery, L. J., Briggs, W. M., Brouwers, E. M., Schornikov, E. I., Stepanova, A., Wood, A. M., Yasuhara, M., and Siu, S.: Arctic Ostracode Database 2020 (AOD2020), NOAA/WDS Paleoclimatology [dataset], https://doi.org/10.25921/grn9-9029, 2021.
Crouch, R. W. and Poag, C. W.: Benthic foraminifera of the Panamanian Province; distribution and origins, J. Foramin. Res., 17, 153–176, https://doi.org/10.2113/gsjfr.17.2.153, 1987.
Culver, S. J. and Buzas, M. A.: Distribution of Recent Benthic Foraminifera off the North American Pacific Coast from Oregon to Alaska, Smithsonian Contributions to the Marine Sciences, no. 26, Smithsonian Institution Press, Washington, D. C., 234 pp., https://doi.org/10.5479/si.01960768.26.1, 1985.
Culver, S. J. and Buzas, M. A.: Distribution of Recent Benthic Foraminifera off the North American Pacific Coast from California to Baja, Smithsonian Contributions to the Marine Sciences, no. 28, Smithsonian Institution Press, Washington, D. C., 634 pp., https://doi.org/10.5479/si.01960768.28.1, 1986.
Culver, S. J. and Buzas, M. A.: Distribution of Recent benthic foraminifera off the Pacific coast of Mexico and Central America, Smithsonian Contributions to the Marine Sciences, no. 30, Smithsonian Institution Press, Washington, D. C., 184 pp., https://doi.org/10.5479/si.01960768.30.1, 1987.
Danovaro, R., Fanelli, E., Aguzzi, J., Billett, D., Carugati, L., Corinaldesi, C., Dell'Anno, A., Gjerde, K., Jamieson, A. J., Kark, S., McClain, C., Levin, L., Levin, N., Ramirez-Llodra, E., Ruhl, H., Smith, C. R., Snelgrove, P. V. R., Thomsen, L., Van Dover, C. L., and Yasuhara, M.: Ecological variables for developing a global deep-ocean monitoring and conservation strategy, Nature Ecology & Evolution, 4, 181–192, https://doi.org/10.1038/s41559-019-1091-z, 2020.
Davies, T. E., Maxwell, S. M., Kaschner, K., Garilao, C., and Ban, N. C.: Large marine protected areas represent biodiversity now and under climate change, Sci. Rep.-UK, 7, 9569, https://doi.org/10.1038/s41598-017-08758-5, 2017.
De, S. and Gupta, A. K.: Deep-sea faunal provinces and their inferred environments in the Indian Ocean based on distribution of Recent benthic foraminifera, Palaeogeogr. Palaeocl., 291, 429–442, https://doi.org/10.1016/j.palaeo.2010.03.012, 2010.
De Miro, M. D. and Gualancañay, E.: Foraminiferos bentonicos de la plataforma continental de la provincia Esmeraldas, Ecuador, Instituto Oceanográfico de la Armada de Ecuador, 12 pp., 1972.
Diz, P., Hernández-Almeida, I., Bernárdez, P., Pérez-Arlucea, M., and Hall, I. R.: Ocean and atmosphere teleconnections modulate east tropical Pacific productivity at late to middle Pleistocene terminations, Earth Planet. Sc. Lett., 493, 82–91, https://doi.org/10.1016/j.epsl.2018.04.024, 2018.
Diz, P., González-Guitián, V., González-Villanueva, R., Ovejero, A., and Hernández-Almeida, I.: BENthic Foraminifera quantitative database from surface sediments of the Eastern Pacific (BENFEP_v1), PANGAEA [dataset], https://doi.org/10.1594/PANGAEA.947086, 2022a.
Diz, P., González-Guitián, V., González-Villanueva, R., Ovejero, A., and Hernández-Almeida, I.: Additional benthic foraminiferal studies in the Eastern Pacific with non-quantitative data, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.947114, 2022b.
Diz, P., González-Guitián, V., González-Villanueva, R., Ovejero, A., and Hernández-Almeida, I.: Video supplement of BENFEP_v1 (1.0), Zenodo [video], https://doi.org/10.5281/zenodo.7472278, 2022c.
Echols, R. J. and Armentrout, J. M.: Holocene Foraminiferal Distribution Patterns on Shelf and Slope, Yakataga–Yakutat Area, Northern Gulf of Alaska, in: Pacific Coast Paleogeography Symposium 4: Quaternary Depositional Environments of the Pacific Coast, Pacific Section, Society for Sedimentary Geology (SEPM), Bakersfield, California, 9 April 1980, 281–303, 1980.
Enge, A. J., Kucera, M., and Heinz, P.: Diversity and microhabitats of living benthic foraminifera in the abyssal Northeast Pacific, Mar. Micropaleontol., 96–97, 84–104, https://doi.org/10.1016/j.marmicro.2012.08.004, 2012.
Enright, S. R., Meneses-Orellana, R., and Keith, I.: The Eastern Tropical Pacific Marine Corridor (CMAR): The Emergence of a Voluntary Regional Cooperation Mechanism for the Conservation and Sustainable Use of Marine Biodiversity Within a Fragmented Regional Ocean Governance Landscape, Frontiers in Marine Science, 8, 674825, https://doi.org/10.3389/fmars.2021.674825, 2021.
Erdem, Z., Schönfeld, J., Rathburn, A. E., Pérez, M.-E., Cardich, J., and Glock, N.: Bottom-water deoxygenation at the Peruvian margin during the last deglaciation recorded by benthic foraminifera, Biogeosciences, 17, 3165–3182, https://doi.org/10.5194/bg-17-3165-2020, 2020.
Erskian, M. G. and Lipps, J. H.: Distribution of Foraminifera in the Russian River Estuary, Northern California, Micropaleontology, 23, 453–469, https://doi.org/10.2307/1485409, 1977.
Finger, K. L.: California foraminiferal micropalaeontology, in: Landmarks in Foraminiferal Micropalaeontology: History and Development, vol. 6, edited by: Bowden, A. J., Gregory, F. J., and Henderson, A. S., Geological Society of London, London, 125–144, https://doi.org/10.1144/TMS6.11, 2013.
Finnegan, S., Anderson, S. C., Harnik, P. G., Simpson, C., Tittensor, D. P., Byrnes, J. E., Finkel, Z. V., Lindberg, D. R., Liow, L. H., Lockwood, R., Lotze, H. K., McClain, C. R., McGuire, J. L., O'Dea, A., and Pandolfi, J. M.: Paleontological baselines for evaluating extinction risk in the modern oceans, Science, 348, 567, https://doi.org/10.1126/science.aaa6635, 2015.
Gardner, J. V., Barron, J. A., Dean, W. E., Heusser, L. E., Poore, R. Z., Quinterno, P., Stone, S. M., and Wilson, C. R.: Quantitative microfossil, sedimentologic, and geochemical data on core L13-81-G138 and surface samples from the continental shelf and slope off Northern California, U. S. Geological Survey, Report 84–369, https://doi.org/10.3133/ofr84369, 1984.
Glock, N., Romero, D., Roy, A. S., Woehle, C., Dale, A. W., Schönfeld, J., Wein, T., Weissenbach, J., and Dagan, T.: A hidden sedimentary phosphate pool inside benthic foraminifera from the Peruvian upwelling region might nucleate phosphogenesis, Geochim. Cosmochim. Ac., 289, 14–32, https://doi.org/10.1016/j.gca.2020.08.002, 2020.
Goineau, A. and Gooday, A. J.: Diversity and spatial patterns of foraminiferal assemblages in the eastern Clarion–Clipperton zone (abyssal eastern equatorial Pacific), Deep-Sea Res. Pt. I, 149, 103036, https://doi.org/10.1016/j.dsr.2019.04.014, 2019.
Golik, A.: Foraminiferal ecology and Holocene history, Gulf of Panama, University of California, San Diego, 198 pp., https://bit.ly/3h8iJYk (last access: November 2022), 1965.
Gooday, A. J., Lejzerowicz, F., Goineau, A., Holzmann, M., Kamenskaya, O., Kitazato, H., Lim, S.-C., Pawlowski, J., Radziejewska, T., Stachowska, Z., and Wawrzyniak-Wydrowska, B.: The Biodiversity and Distribution of Abyssal Benthic Foraminifera and Their Possible Ecological Roles: A Synthesis Across the Clarion-Clipperton Zone, Frontiers in Marine Science, 8, 634726, https://doi.org/10.3389/fmars.2021.634726, 2021.
Harman, R. A.: Distribution of foraminifera in the Santa Barbara Basin, California, Micropaleontology, 10, 81–96, https://doi.org/10.2307/1484628, 1964.
Hayward, B. W., Le Coze, F., Vachard, D., and Gross, O.: World Foraminifera Database, https://www.marinespecies.org/foraminifera, last access: 8 December 2022.
Heinz, P., Ruschmeier, W., and Hemleben, C.: Live benthic foraminiferal Assemblages at the Pacific continental margin of Costa Rica and Nicaragua, J. Foramin. Res., 38, 215–227, https://doi.org/10.2113/gsjfr.38.3.215, 2008.
Hernández-Almeida, I., Boltovskoy, D., Kruglikova, S. B., and Cortese, G.: A new radiolarian transfer function for the Pacific Ocean and application to fossil records: Assessing potential and limitations for the last glacial-interglacial cycle, Global Planet. Change, 190, 103186, https://doi.org/10.1016/j.gloplacha.2020.103186, 2020.
Hromic, T.: Foraminíferos Bentónicos recientes del Estrecho de Magallanes, y canales australes chilenos CIMAR 3 FIORDOS (52∘–56∘ S), Anales Instituto Patagonia (Chile), 39, 17–32, 2011.
Hromic, T., Ishman, S., and Silva, N.: Benthic foraminiferal distributions in Chilean fjords: 47∘ S to 54∘ S, Mar. Micropaleontol., 59, 115–134, https://doi.org/10.1016/j.marmicro.2006.02.001, 2006.
Huang, H.-H. M., Yasuhara, M., Horne, D. J., Perrier, V., Smith, A. J., and Brandão, S. N.: Ostracods in databases: State of the art, mobilization and future applications, Mar. Micropaleontol., 174, 102094, https://doi.org/10.1016/j.marmicro.2022.102094, 2022.
Ingle, J. C. and Keller, G.: Benthic foraminiferal biofacies of the eastern Pacific margin between 40∘ S and 32∘ N, in: Pacific Coast Paleogeography Symposium 4: Quaternary Depositional Environments of the Pacific Coast, Pacific Section, Society for Sedimentary Geology (SEPM), Bakersfield, California, 9 April 1980, 341–355, 1980.
Ingle, J. C., Keller, G., and Kolpack, R. L.: Benthic foraminiferal biofacies, sediments and water masses of the Southern Perú-Chile Trench Area, Southeastern Pacific Ocean, Micropaleontology, 26, 113–150, https://doi.org/10.2307/1485435, 1980.
Jones, G. D. and Ross, C. A.: Seasonal Distribution of Foraminifera in Samish Bay, Washington, J. Paleontol., 53, 245–257, 1979.
Jonkers, L., Hillebrand, H., and Kucera, M.: Global change drives modern plankton communities away from the pre-industrial state, Nature, 570, 372–375, https://doi.org/10.1038/s41586-019-1230-3, 2019.
Jorissen, F., Nardelli, M. P., Almogi-Labin, A., Barras, C., Bergamin, L., Bicchi, E., El Kateb, A., Ferraro, L., McGann, M., Morigi, C., Romano, E., Sabbatini, A., Schweizer, M., and Spezzaferri, S.: Developing Foram-AMBI for biomonitoring in the Mediterranean: Species assignments to ecological categories, Mar. Micropaleontol., 140, 33–45, https://doi.org/10.1016/j.marmicro.2017.12.006, 2018.
Jorissen, F. J., Fontanier, C., and Thomas, E.: Chapter Seven Paleoceanographical Proxies Based on Deep-Sea Benthic Foraminiferal Assemblage Characteristics, in: Developments in Marine Geology, vol. 1, edited by: Hillaire-Marcel, C. and De Vernal, A., Elsevier, 263–325, https://doi.org/10.1016/s1572-5480(07)01012-3, 2007.
Karstensen, J., Stramma, L., and Visbeck, M.: Oxygen minimum zones in the eastern tropical Atlantic and Pacific oceans, Prog. Oceanogr., 77, 331–350, https://doi.org/10.1016/j.pocean.2007.05.009, 2008.
Kidwell, S. M.: Biology in the Anthropocene: Challenges and insights from young fossil records, P. Natl. Acad. Sci. USA, 112, 4922–4929, https://doi.org/10.1073/pnas.1403660112, 2015.
Krumhardt, K. M., Lovenduski, N. S., Iglesias-Rodriguez, M. D., and Kleypas, J. A.: Coccolithophore growth and calcification in a changing ocean, Prog. Oceanogr., 159, 276–295, https://doi.org/10.1016/j.pocean.2017.10.007, 2017.
Lankford, R. R. and Phleger, F. B.: Foraminifera from the nearshore turbulent zone, western North America, J. Foramin. Res., 3, 101–132, https://doi.org/10.2113/gsjfr.3.3.101, 1973.
Leblanc, K., Arístegui, J., Armand, L., Assmy, P., Beker, B., Bode, A., Breton, E., Cornet, V., Gibson, J., Gosselin, M.-P., Kopczynska, E., Marshall, H., Peloquin, J., Piontkovski, S., Poulton, A. J., Quéguiner, B., Schiebel, R., Shipe, R., Stefels, J., van Leeuwe, M. A., Varela, M., Widdicombe, C., and Yallop, M.: A global diatom database – abundance, biovolume and biomass in the world ocean, Earth Syst. Sci. Data, 4, 149–165, https://doi.org/10.5194/essd-4-149-2012, 2012.
Liu, X.: The effect of an oxygen minimum zone on benthic foraminifera on a seamount near the East Pacific Rise, Department of Geology, The Florida State University College of Arts and Sciences, 104 pp., https://bit.ly/3KpszRU (last access: June 2021), 2001.
Liu, Z. and Herbert, T. D.: High-latitude influence on the eastern equatorial Pacific climate in the early Pleistocene epoch, Nature, 427, 720–723, 2004.
Loubere, P.: Quantitative estimation of surface ocean productivity and bottom water oxygen concentration using benthic foraminifera, Paleoceanography, 9, 723–737, https://doi.org/10.1029/94PA01624, 1994.
Mackensen, A. and Douglas, R. G.: Down-core distribution of live and dead deep-water benthic foraminifera in box cores from the Weddell Sea and the California continental borderland, Deep-Sea Res., 36, 879–900, https://doi.org/10.1016/0198-0149(89)90034-4, 1989a.
Mackensen, A. and Douglas, R. G.: Down-core distribution of live and dead benthic foraminifera in deep sea sediments from the Weddell Sea and the Californian continental borderland, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.710895, 1989b.
Mallon, J.: Benthic foraminifera of the Peruvian & Ecuadorian continental margin, Dissertation, Universität Kiel, 279 pp., https://macau.uni-kiel.de/servlets/MCRFileNodeServlet/dissertation_derivate_00004121/Mallon_benthic__foraminifera.pdf (last access: November 2022), 2011.
Marret, F., Bradley, L., de Vernal, A., Hardy, W., Kim, S.-Y., Mudie, P., Penaud, A., Pospelova, V., Price, A. M., Radi, T., and Rochon, A.: From bi-polar to regional distribution of modern dinoflagellate cysts, an overview of their biogeography, Mar. Micropaleontol., 159, 101753, https://doi.org/10.1016/j.marmicro.2019.101753, 2020.
Martin, L. N.: Observations on Living Foraminifera from the intertidal zone of Monterey Bay, California, Biological Sciences, Stanford, 66 pp., VI plates, https://stacks.stanford.edu/file/dx798gr0222/dx798gr0222.pdf (last access: November 2021), 1932.
Martin, R. A., Nesbitt, E. A., and Martin, D. E.: Distribution of foraminifera in Puget Sound, Western Washington, U. S. A., J. Foramin. Res., 43, 291–304, https://doi.org/10.2113/gsjfr.43.3.291, 2013.
McGann, M.: Historical and modern distributions of benthic foraminifers on the continental shelf of Monterey Bay, California, Mar. Geol., 181, 115–156, https://doi.org/10.1016/S0025-3227(01)00264-X, 2002.
McGann, M. L., Schmieder, R. W., and Loncke, L.-P.: Shallow-Water Foraminifera and Other Microscopic Biota of Clipperton Island, Tropical Eastern Pacific, Atoll Research Bulletin, no. 626, Smithsonian Scholarly Press, https://doi.org/10.5479/si.10329962, 2019.
McGlasson, R. H.: Foraminiferal Biofacies around Santa Catalina Island, California, Micropaleontology, 5, 217–240, https://doi.org/10.2307/1484211, 1959.
Mekik, F. and Anderson, R.: Is the core top modern? Observations from the eastern equatorial Pacific, Quaternary Sci. Rev., 186, 156–168, https://doi.org/10.1016/j.quascirev.2018.01.020, 2018.
Morin, R. W.: Foraminiferal Populations in the Santa Barbara Channel: Offshore Species, in: Biological and Oceanographical Survey of the Santa Barbara Channel Oil Spill, 1969–1970, Volume II Physical, Chemical and Geological Studies, edited by: Kolpack, R. L., Allen Hancock Foundation, University of Southern California, 218–275, 1971.
Moyer, D. A.: Shallow Water Foraminifera from off Point Firmin, San Pedro, California, Micropaleontology Bulletin, 11, 5–10, 1929.
Murray, J. W.: The enigma of the continued use of total assemblages in ecological studies of benthic foraminifera, J. Foramin. Res., 30, 244–245, https://doi.org/10.2113/0300244, 2000.
Murray, J. W.: Ecology and palaeoecology of benthic foraminifera, Routledge, https://doi.org/10.4324/9781315846101, 2006.
Murray, J. W.: Biodiversity of living benthic foraminifera: How many species are there?, Mar. Micropaleontol., 64, 163–176, https://doi.org/10.1016/j.marmicro.2007.04.002, 2007.
Murray, J. W.: Living benthic foraminifera: biogeographical distributions and the significance of rare morphospecies, J. Micropalaeontol., 32, 1–58, https://doi.org/10.1144/jmpaleo2012-010, 2013.
Natland, M. L.: The temperature-and depth-distribution of some recent and fossil foraminifera in the southern California region, Bulletin, Scripps Institution of Oceanography, 3, 225–231, 1933.
Nienstedt, J. C.: Biogeographic distribution of recent benthic foraminifera near the East Pacific Rise, The Florida State University College of Arts and Sciences, 149 pp., https://bit.ly/36xhUpT (last access: June 2021), 1986.
Palmer, H. M., Hill, T. M., Myhre, S. E., Roopnarine, P. R., Reyes, K. R., and Donnenfield, J. T.: San Diego Margin Benthic Foraminiferal Assemblages from Late Holocene, NOAA National Centers for Enviromental Information [data set], https://doi.org/10.25921/c522-4h11, 2019.
Palmer, H. M., Hill, T. M., Roopnarine, P. D., Myhre, S. E., Reyes, K. R., and Donnenfield, J. T.: Southern California margin benthic foraminiferal assemblages record recent centennial-scale changes in oxygen minimum zone, Biogeosciences, 17, 2923–2937, https://doi.org/10.5194/bg-17-2923-2020, 2020.
Patarroyo, G. D. and Martínez, J. I.: Late quaternary sea bottom conditions in the southern Panama basin, Eastern Equatorial Pacific, J. S. Am. Earth Sci., 63, 346–359, https://doi.org/10.1016/j.jsames.2015.07.010, 2015.
Patarroyo, G. D. and Martinez, J. I.: Composition and diversity patterns of deep sea benthic foraminifera from the Panama basin, eastern equatorial Pacific, Deep-Sea Res. Pt. I, 169, 103470, https://doi.org/10.1016/j.dsr.2021.103470, 2021.
Patterson, R. T., Guilbault, J.-P., and Thomson, R. E.: Oxygen level control on foraminiferal distribution in Effingham inlet, Vancouver island, British Columbia, Canada, J. Foramin. Res., 30, 321–335, https://doi.org/10.2113/0300321, 2000.
Pedersen, T. L.: ggforce: Accelerating 'ggplot2', R package version 0.4.1., https://CRAN.R-project.org/package=ggforce (last access: December 2022), 2022.
Perez-Cruz, L. L. and Machain-Castillo, M. L.: Benthic foraminifera of the oxygen minimum zone, continental shelf of the Gulf of Tehuantepec, Mexico, J. Foramin. Res., 20, 312–325, https://doi.org/10.2113/gsjfr.20.4.312, 1990.
Pettit, L. R., Hart, M. B., Medina-Sánchez, A. N., Smart, C. W., Rodolfo-Metalpa, R., Hall-Spencer, J. M., and Prol-Ledesma, R. M.: Benthic foraminifera show some resilience to ocean acidification in the northern Gulf of California, Mexico, Mar. Pollut. Bull., 73, 452–462, https://doi.org/10.1016/j.marpolbul.2013.02.011, 2013a.
Pettit, L. R., Hart, M. B., Medina-Sánchez, A. N., Smart, C. W., Rodolfo-Metalpa, R., Hall-Spencer, J. and Prol-Ledesma, R.: (Table 2) Live (stained) benthic foraminifera from stations in the northern Gulf of California, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.816336, 2013b.
Pettit, L. R., Hart, M. B., Medina-Sánchez, A. N., Smart, C. W., Rodolfo-Metalpa, R., Hall-Spencer, J. and Prol-Ledesma, R.: (Table 3) Counts of dead (not stained) benthic foraminifera from stations in the northern Gulf of California, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.816337, 2013c.
Phleger, F. B.: Patterns of Living Benthonic Foraminifera, Gulf of California, in: Marine Geology of the Gulf of California: a symposium, edited by: van Andel, T. H., and Shor Jr., G. G., American Association of Petroleum Geologists, 377–394, https://doi.org/10.1306/M3359C14, 1964.
Phleger, F. B.: Depth patterns of benthonic foraminifera in the Eastern Pacific, Prog. Oceanogr., 3, 273–287, https://doi.org/10.1016/0079-6611(65)90023-6, 1965.
Pisias, N. G., Mix, A. C., and Heusser, L.: Millennial scale climate variability of the northeast Pacific Ocean and northwest North America based on radiolaria and pollen, Quaternary Sci. Rev., 20, 1561–1576, https://doi.org/10.1016/S0277-3791(01)00018-X, 2001.
Praetorius, S. K., Condron, A., Mix, A. C., Walczak, M. H., McKay, J. L., and Du, J.: The role of Northeast Pacific meltwater events in deglacial climate change, Science Advances, 6, eaay2915, https://doi.org/10.1126/sciadv.aay2915, 2020.
R Core Team: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/, last access: September 2022.
Reiter, M.: Seasonal Variations in Intertidal Foraminifera of Santa Monica Bay, California, J. Paleontol., 33, 606–630, 1959.
Resig, J. M.: Ecology of Foraminifera of the Santa Cruz Basin, California, Micropaleontology, 4, 287–308, https://doi.org/10.2307/1484288, 1958.
Resig, J. M.: Foraminiferal ecology around ocean outfalls off southern California, Proceedings of the First International Conference on Waste Disposal in the Marine Environment, University of California, Berkeley, 22–25 July 1959, 104–121, 1960.
Resig, J. M.: Biogeography of benthic foraminifera of the northern Nazca plate and adjacent continental margin, in: Nazca Plate: Crustal Formation and Andean Convergence, edited by: Kulm, L. V. D., Dymond, J., Dasch, E. J., Hussong, D. M., and Roderick, R., Geological Society of America, 619–666, https://doi.org/10.1130/MEM154-p619, 1981.
Saidova, Kh. M.: On large-scale facies confinement of deep-sea benthic foraminifera, Oceanol. Acta, 14, 534–540, 1974.
Schönfeld, J.: History and development of methods in Recent benthic foraminiferal studies, J. Micropalaeontol., 31, 53–72, https://doi.org/10.1144/0262-821X11-008, 2012.
Scott, D. B., Mudie, P. J., and Bradshaw, J. S.: Benthonic foraminifera of three southern Californian lagoons; ecology and Recent stratigraphy, J. Foramin. Res., 6, 59–75, https://doi.org/10.2113/gsjfr.6.1.59, 1976.
Sejrup, H. P., Birks, H. J. B., Klitgaard Kristensen, D., and Madsen, H.: Benthonic foraminiferal distributions and quantitative transfer functions for the northwest European continental margin, Mar. Micropaleontol., 53, 197–226, https://doi.org/10.1016/j.marmicro.2004.05.009, 2004.
Sharon, Belanger, C., Du, J., and Mix, A.: Reconstructing paleo-oxygenation for the last 54 000 years in the Gulf of Alaska using cross-validated benthic foraminiferal and geochemical records, Paleoceanography and Paleoclimatology, 36, e2020PA003986, https://doi.org/10.1029/2020PA003986, 2020.
Sherman, K.: The Large Marine Ecosystem Concept: Research and Management Strategy for Living Marine Resources, Ecol. Appl., 1, 350–360, https://doi.org/10.2307/1941896, 1991.
Siccha, M. and Kucera, M.: ForCenS, a curated database of planktonic foraminifera census counts in marine surface sediment samples, Scientific Data, 4, 170109, https://doi.org/10.1038/sdata.2017.109, 2017.
Smith, P. B.: Quantitative and qualitative analysis of the family Bolivinidae, Geological Survey Professional Paper 429-A, United States Goverment Printing Office, Washington, https://doi.org/10.3133/pp429A, 1963.
Smith, P. B.: Ecology of benthonic species, Geological Survey Professional Paper 429-B, United States Goverment Printing Office, Washington, https://doi.org/10.3133/pp429B, 1964.
Smith, P. B.: Foraminifera of the North Pacific Ocean, Geological Survey Professional Paper 766, United States Goverment Printing Office, Washington, https://doi.org/10.3133/pp766, 1973.
Snyder, S. W., Hale, W. R., and Kontrovitz, M.: Distributional Patterns of Modern Benthic Foraminifera on the Washington Continental Shelf, Micropaleontology, 36, 245–258, https://doi.org/10.2307/1485508, 1990.
Stuecker, M. F.: Revisiting the Pacific Meridional Mode, Sci. Rep.-UK, 8, 3216, https://doi.org/10.1038/s41598-018-21537-0, 2018.
Sweetman, A. K., Thurber, A. R., Smith, C. R., Levin, L. A., Mora, C., Wei, C.-L., Gooday, A. J., Jones, D. O. B., Rex, M., Yasuhara, M., Ingels, J., Ruhl, H. A., Frieder, C. A., Danovaro, R., Würzberg, L., Baco, A., Grupe, B. M., Pasulka, A., Meyer, K. S., Dunlop, K. M., Henry, L.-A., and Roberts, J. M.: Major impacts of climate change on deep-sea benthic ecosystems, Elementa: Science of the Anthropocene, 5, 4, https://doi.org/10.1525/elementa.203, 2017.
Takata, H., Yoo, C. M., Kim, H. J., and Khim, B.-K.: Latitudinal change in benthic foraminiferal fauna by ITCZ movement along the ∼131∘ W transect in the equatorial Pacific Ocean, Ocean Sci. J., 51, 655–663, https://doi.org/10.1007/s12601-016-0048-2, 2016.
Tapia, R., Ho, S. L., Núñez-Ricardo, S., Marchant, M., Lamy, F., and Hebbeln, D.: Increased marine productivity in the southern Humboldt Current System during MIS 2–4 and 10–11, Paleoceanography and Paleoclimatology, 36, e2020PA004066, https://doi.org/10.1029/2020PA004066, 2021.
Tavera Martínez, L., Marchant, M., Muñoz, P., and Abdala Díaz, R. T.: Spatial and Vertical Benthic Foraminifera Diversity in the Oxygen Minimum Zone of Mejillones Bay, Northern Chile, Frontiers in Marine Science, 9, 821564, https://doi.org/10.3389/fmars.2022.821564, 2022.
Tetard, M., Licari, L., and Beaufort, L.: Oxygen history off Baja California over the last 80 kyr: A new foraminiferal-based record, Paleoceanography, 32, 246–264, https://doi.org/10.1002/2016pa003034, 2017.
Tetard, M., Licari, L., Ovsepyan, E., Tachikawa, K., and Beaufort, L.: Toward a global calibration for quantifying past oxygenation in oxygen minimum zones using benthic Foraminifera, Biogeosciences, 18, 2827–2841, https://doi.org/10.5194/bg-18-2827-2021, 2021.
Todd, R. and Low, D.: Recent foraminifera from the Gulf of Alaska and southeastern Alaska, Report 573A, https://doi.org/10.3133/pp573A, 1967.
Uchimura, H., Nishi, H., Takashima, R., Kuroyanagi, A., Yamamoto, Y., and Kutterolf, S.: Distribution of Recent Benthic Foraminifera off Western Costa Rica in the Eastern Equatorial Pacific Ocean, Paleontol. Res., 21, 380–396, https://doi.org/10.2517/2017PR003, 2017.
Uchio, T.: Ecology of living benthonic foraminifera from the San Diego, California, Area Cushman Foundation for Foraminiferal Research, Special Publication no. 5, 1–81, ISBN 9781970168044, 1960.
United Nations Educational, Scientific and Cultural Organization (UNESCO): World Heritage List, https://whc.unesco.org/en/list/, last access: May 2022.
van Morkhoven, F. P. C. M., Berggren, W. A., and Edwards, A. S.: Cenozoic cosmopolitan deep-water benthic foraminifera, Bull. Centres Rech. Explor.-Prod. Elf-Aquitaine, Mem. 11, Pau, 421 pp., ISSN 0181-0901, 1986.
Venturelli, R.: Abundance data of benthic foraminifera in sediment core tops sampled during the NH1108 cruise to Southern California Bight in July 2011, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.893323, 2018.
Venturelli, R. A., Rathburn, A. E., Burkett, A. M., and Ziebis, W.: Epifaunal Foraminifera in an Infaunal World: Insights Into the Influence of Heterogeneity on the Benthic Ecology of Oxygen-Poor, Deep-Sea Habitats, Frontiers in Marine Science, 5, 344, https://doi.org/10.3389/fmars.2018.00344, 2018.
Violanti, D., Loi, B., and Melis, R.: Distribution of Recent Foraminifera from Strair of Magellan. First quantitative data, Bolletino Museo Regionale di Scienze Naturali Torino, 17, 511–539, 2000.
Walch, A.: Recent abyssal benthic foraminifera from the Eastern Equatorial Pacific, Southern California, 117 pp., https://bit.ly/35yR9RL (last access: August 2021), 1978.
Walczak, M. H., Mix, A. C., Cowan, E. A., Fallon, S., Fifield, L. K., Alder, J. R., Du, J., Haley, B., Hobern, T., Padman, J., Praetorius, S. K., Schmittner, A., Stoner, J. S., and Zellers, S. D.: Phasing of millennial-scale climate variability in the Pacific and Atlantic Oceans, Science, 370, 716–720, https://doi.org/10.1126/science.aba7096, 2020.
Walton, W. R.: Techniques for recognition of living foraminifera, Contributions from the Cushman Foundation for Foraminiferal Research, 3, 56–60, 1952.
Walton, W. R.: Ecology of Living Benthonic Foraminifera Todos Santos Bay, Baja California, University of California, Los Angeles, https://bit.ly/3MmCtWm (last access: February 2021), 1954.
Walton, W. R.: Ecology of Living Benthonic Foraminifera, Todos Santos Bay, Baja California, J. Paleontol., 29, 952–1018, http://www.jstor.org/stable/1300447 (last access: March 2022), 1955.
Watkins, J. G.: Foraminiferal Ecology around the Orange County, California, Ocean Sewer Outfall, Micropaleontology, 7, 199–206, https://doi.org/10.2307/1484279, 1961.
Weaver, P. P. E. and Schultheiss, P. J.: Current methods for obtaining, logging and splitting marine sediment cores, Mar. Geophys. Res., 12, 85–100, https://doi.org/10.1007/BF00310565, 1990.
Wickham, H., Averick, M., Bryan, J., Chang, W., D'Agostino McGowan, L., Francois, R., Grolemund, G., Hayes, A., Henry, L., and Hester, J.: Welcome to the Tidyverse, The Journal of Open Source Software, 4, 6, https://doi.org/10.21105/joss.01686, 2019.
Wilkinson, M. D., Dumontier, M., Aalbersberg, I. J., Appleton, G., Axton, M., Baak, A., Blomberg, N., Boiten, J.-W., da Silva Santos, L. B., Bourne, P. E., Bouwman, J., Brookes, A. J., Clark, T., Crosas, M., Dillo, I., Dumon, O., Edmunds, S., Evelo, C. T., Finkers, R., Gonzalez-Beltran, A., Gray, A. J. G., Groth, P., Goble, C., Grethe, J. S., Heringa, J., 't Hoen, P. A. C., Hooft, R., Kuhn, T., Kok, R., Kok, J., Lusher, S. J., Martone, M. E., Mons, A., Packer, A. L., Persson, B., Rocca-Serra, P., Roos, M., van Schaik, R., Sansone, S.-A., Schultes, E., Sengstag, T., Slater, T., Strawn, G., Swertz, M. A., Thompson, M., van der Lei, J., van Mulligen, E., Velterop, J., Waagmeester, A., Wittenburg, P., Wolstencroft, K., Zhao, J., and Mons, B.: The FAIR Guiding Principles for scientific data management and stewardship, Scientific Data, 3, 160018, https://doi.org/10.1038/sdata.2016.18, 2016.
Wollenburg, J. E. and Kuhnt, W.: The response of benthic foraminifers to carbon flux and primary production in the Arctic Ocean, Mar. Micropaleontol., 40, 189–231, https://doi.org/10.1016/S0377-8398(00)00039-6, 2000.
Yasuhara, M., Hunt, G., Breitburg, D., Tsujimoto, A., and Katsuki, K.: Human-induced marine ecological degradation: micropaleontological perspectives, Ecol. Evol., 2, 3242–3268, https://doi.org/10.1002/ece3.425, 2012.
Yasuhara, M., Rabalais, N. N., Conley, D. J., and Gutiérrez, D.: Palaeo-records of histories of deoxygenation and its ecosystem impact, in: Ocean deoxygenation: Everyone's problem – Causes, impacts, consequences and solutions, edited by: Laffoley, D. and Baxter, J. M., IUCN, Gland, Switzerland, 213–224, https://doi.org/10.2305/IUCN.CH.2019.13.en, 2019.
Yasuhara, M., Wei, C.-L., Kucera, M., Costello, M. J., Tittensor, D. P., Kiessling, W., Bonebrake, T. C., Tabor, C. R., Feng, R., Baselga, A., Kretschmer, K., Kusumoto, B., and Kubota, Y.: Past and future decline of tropical pelagic biodiversity, P. Natl. Acad. Sci. USA, 117, 12891, https://doi.org/10.1073/pnas.1916923117, 2020.
Young, J., Geisen, M., Cros, L., Kleijne, A., Sprengel, C., Probert, I., and Østergaard, J.: A guide to extant coccolithophore taxonomy, Journal of Nannoplankton Research, Special Issue, 1, 1–132, 2003.
Zalesny, E. R.: Foraminiferal Ecology of Santa Monica Bay, California, Micropaleontology, 5, 101–126, https://doi.org/10.2307/1484158, 1959.
Zapata, J., Zapata, C., and Gutiérrez, A.: Foraminíferos bentónicos recientes del sur de Chile, Gayana Zoologia, 59, 23–40, 1995.
- Abstract
- Introduction
- Methods
- Results and discussion
- Recommendations for archiving benthic foraminifera quantitative data
- Data availability
- Conclusions
- Appendix A
- Appendix B
- Appendix C
- Video supplement
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- Methods
- Results and discussion
- Recommendations for archiving benthic foraminifera quantitative data
- Data availability
- Conclusions
- Appendix A
- Appendix B
- Appendix C
- Video supplement
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement