the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
A comprehensive dataset of microbial abundance, dissolved organic carbon, and nitrogen in Tibetan Plateau glaciers
Yongqin Liu
Pengcheng Fang
Bixi Guo
Pengfei Liu
Guannan Mao
Baiqing Xu
Shichang Kang
Glaciers are recognized as a biome dominated by microorganisms and a reservoir of organic carbon and nutrients. Global warming remarkably increases glacier melting rate and runoff volume, which have significant impacts on the carbon and nitrogen cycles in downstream ecosystems. The Tibetan Plateau (TP), dubbed “the water tower of Asia”, owns the largest mountain glacial area at mid- and low-latitudes. However, limited data on the microbial abundance, organic carbon, and nitrogen in TP glaciers are available in the literature, which severely hinders our understanding of the regional carbon and nitrogen cycles. This work presents a new dataset on microbial abundance, dissolved organic carbon (DOC), and total nitrogen (TN) for TP glaciers. In this dataset, there are 5409 records from 12 glaciers for microbial abundance in ice cores and snow pits, and 2532 records from 38 glaciers for DOC and TN in the ice core, snow pit, surface ice, surface snow, and proglacial runoff. These glaciers are located across diverse geographic and climatic regions, where the multiyear average air temperature ranges from −13.4 to 2.9 ∘C and the multiyear average precipitation ranges from 76.9 to 927.8 mm. This makes the constructed dataset qualified for large-scale studies across the TP. To the best of our knowledge, this is the first dataset of microbial abundance and TN in TP glaciers and also the first dataset of DOC in ice cores of the TP. This new dataset provides important information for studies on carbon and nitrogen cycles in glacial ecosystems, and is especially valuable for the assessment of potential impacts of glacier retreat on downstream ecosystems under global warming. The dataset is available from the National Tibetan Plateau/Third Pole Environment Data Center (https://doi.org/10.11888/Cryos.tpdc.271841; Liu, 2021).
- Article
(12010 KB) - Full-text XML
-
Supplement
(58 KB) - BibTeX
- EndNote
The glacier is regarded as an extreme environment, featured by sustained low temperature, lack of nutrients, and strong radiation. Abundant and active microorganisms play important roles in the biogeochemical cycling in glacier ecosystems (Liu et al., 2016a; Smith et al., 2017; Irvine-Fynn et al., 2021), and the glacier is a reservoir of microorganisms, organic carbon, and nitrogen (Anesio and Laybourn-Parry, 2012; Hood et al., 2015). Carbon and nitrogen in glaciers could be originated from atmospheric deposition or microbial activities (Anesio et al., 2017; Hodson et al., 2008). These nutrients are vital for downstream aquatic ecosystems (Hodson et al., 2008; Dubnick et al., 2017), especially for the oligotrophic proglacial streams and lakes (Hood et al., 2015). Glaciers are also the species pools for downstream aquatic ecosystems and substantially influence the microbial functions in downstream ecosystems. It has been reported that glacier-originated bacteria can contribute up to 20 % of the microbial community in the proglacial stream 20 km downstream (Liu et al., 2021), and glacier retreat can change the fungal abundance and cellulose decomposition rate in mountain rivers (Fell et al., 2021). Under global climate warming, glaciers are melting at an accelerating rate: the carbon, nitrogen, and microorganisms stored in glaciers will be released in glacier runoff, and may alter the carbon and nitrogen cycling in downstream ecosystems (Hood et al., 2009; Singer et al., 2012; Fellman et al., 2015; Wadham et al., 2016).
The Tibetan Plateau (TP), dubbed “the water tower of Asia”, owns the largest mountain glacial area at mid- and low-latitudes (Yao et al., 2012). Glaciers on the TP are the sources of several large rivers in Asia and are thus of great importance for downstream regions (Yao et al., 2019). However, due to harsh climate conditions and logistic difficulties, there have been limited reports of microorganisms, organic carbon, and nitrogen in TP glaciers, especially in ice cores. Microbial abundance in ice cores is only available in three TP glaciers (i.e., Puruoganri, East Rongbuk, and Malan; Yao et al., 2006; Zhang et al., 2008, 2010) using the epifluorescence microscopy method, and there is as yet no publicly available dataset on the dissolved organic carbon (DOC) and total nitrogen (TN) in ice cores from the TP. Even for the surface ice and snow, the data on microbial abundances, DOC, or TN have only been reported in less than 10 glaciers (Hood et al., 2015; Liu et al., 2016b; Hu et al., 2018; Yan et al., 2020; Kang et al., 2022). Data scarcity has significantly hindered our understanding of the biogeochemical cycle in TP glaciers and downstream ecosystems.
In this study, we aimed to construct a comprehensive dataset of microbial abundance, DOC, and TN in TP glaciers through extensive field sampling and experimental measurements. In this dataset, there are 5409 records from 12 glaciers for microbial abundance in ice cores and snow pits, and 2532 records from 38 glaciers for DOC and TN in the ice core, snow pit, surface ice, surface snow, and proglacial runoff. This dataset can provide fundamental data for analyzing the storage, spatial pattern, and drivers of glacier carbon and nitrogen on the TP. It can also facilitate the investigations of glacier biogeochemical cycles and evaluate the impact of glacier retreat on downstream ecosystems.
The TP covers an area of about 2.5 million km2, with an average altitude of more than 4000 m above sea level (a.s.l.). The climate over the TP is primarily influenced by the interaction between the Indian monsoon and the westerly wind (Tian et al., 2001). The TP and its surrounding regions contain the largest number of glaciers outside the poles with an area of about 47 000 km2 (Yao et al., 2012). These glaciers are important water resources for downstream areas and play a crucial role in regional water supply (Immerzeel et al., 2010). The glaciers on the TP are mainly distributed in the Kunlun, Nyainqntanglha, Himalaya, and Karakoram mountains, and most glaciers are located between 4500 and 6500 m a.s.l. (Liu et al., 2015). The glaciers can be divided into three types (Shi and Liu, 2000): monsoonal temperate glaciers (mainly distributed in the southeast of the TP), subcontinental glaciers (mainly distributed in the northeast and southern margin of the TP), and the continental glaciers (mainly distributed in the west of the TP). Most of the TP glaciers, except those in the Karakoram region, are experiencing rapid retreat under climate warming (Yao et al., 2012; Wang et al., 2021).
3.1 Sample distribution
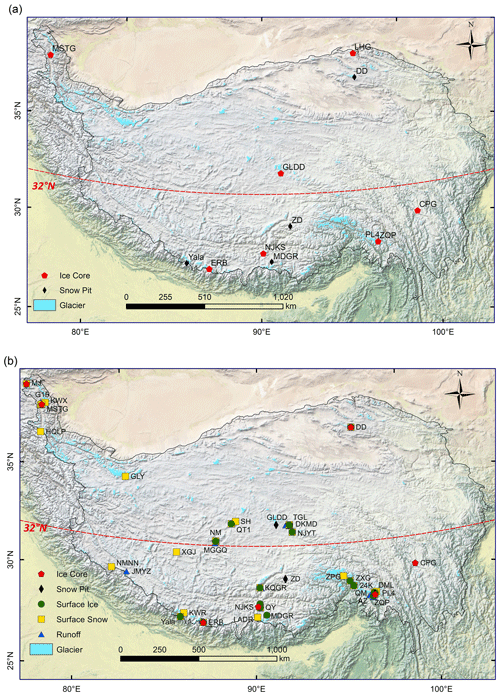
In this study, 5409 microbial abundance data are distributed across 12 glaciers across the TP (Fig. 1a), including 5210 ice core samples from 7 glaciers and 199 snow-pit samples from 7 glaciers. For DOC and TN, 2532 samples from 38 glaciers were collected as shown in Fig. 1b, including 1625 ice core samples from 7 glaciers, 180 surface ice samples from 17 glaciers, 100 snow-pit samples from 4 glaciers, 254 surface snow samples from 28 glaciers, and 397 proglacial runoff samples from 16 glaciers.
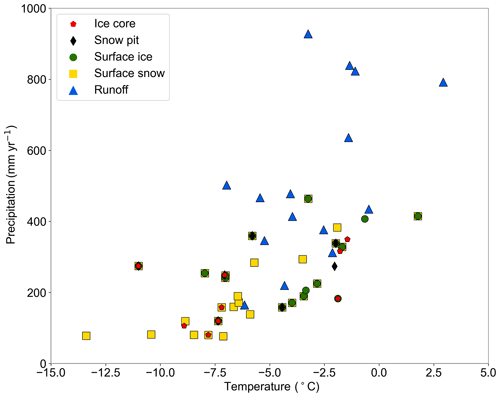
The sampled glaciers cover diverse climate conditions. The multiyear average air temperature ranged from −13.4 (the Guliya glacier) to 2.9 ∘C (the Zhuxigou glacier), and the multiyear average precipitation ranged from 76.9 mm (the No. 15 glacier) to 927.8 mm (the 24K glacier). The temperature and precipitation of most glaciers over the TP are within these ranges, which makes the dataset comprehensive and representative.
3.2 Sample distribution
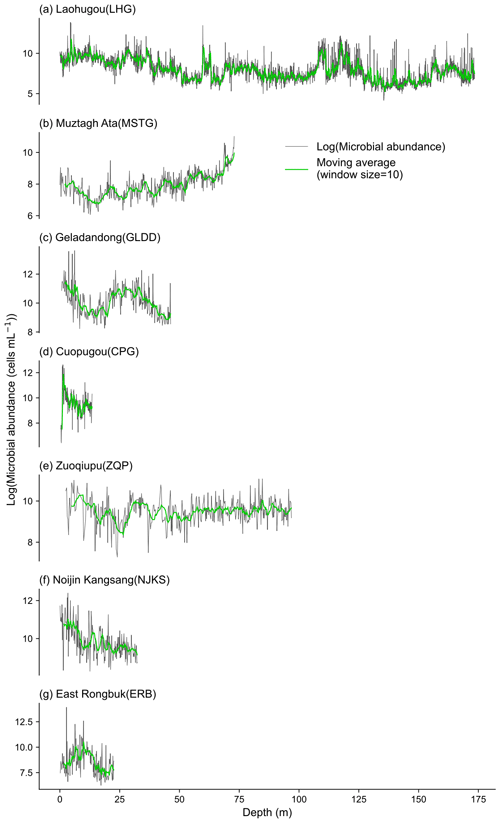
Ice cores were drilled from the accumulation zones of nine glaciers to depths from 11 to 173 m (Fig. 1) and then transported frozen to the laboratory. Both microbial abundance and DOC/TN data were measured for samples from five glaciers (i.e., Muztagh Ata (MSTG), Cuopugou (CPG), Zuoqiupu (ZQP), Noijin Kangsang (NJKS), and East Rongbuk (ERB)); for samples from the Laohugou (LHG) and Geladandong (GLDD) glacier, only microbial abundance was measured; for samples from Muji (MJ) and Dunde (DD) glaciers, only DOC and TN were measured. The MJ, MSTG, LHG, DD, and GLDD glaciers are mainly influenced by the westerly wind, while the CPG, ZQP, NJKS, and ERB glaciers are strongly influenced by the Indian monsoon.
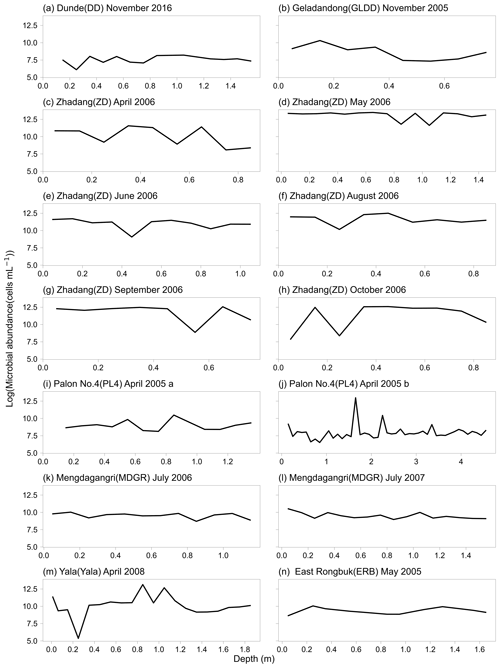
Snow pits were dug at the accumulation zones of seven glaciers, and snow was sampled from top to bottom at 5 or 10 cm intervals in each pit using a pre-sterilized steel scoop. Microbial abundance, DOC, and TN concentrations were measured for samples from GLDD, Zhadang (ZD), Palon No. 4 (PL4), and ERB glacier, while only microbial abundance was measured for samples from DD, Mengdagangri (MDGR), and Yala glacier. More specifically, 14 snow pits were analyzed for microbial abundance measurement, including 6 at ZD glacier during April, May, June, August, September, and October in 2006, 2 at the MDGR glacier in 2006 and 2007, 2 at different altitudes in the PL4 glacier, and 1 for each at the DD, GLDD, ERB, and Yala glacier, respectively. Four snow pits were sampled for DOC and TN concentration measurements with one snow pit in each glacier among GLDD, ZD, PL4, and ERB.
Surface snow (top 10 cm) was sampled using a pre-sterilized steel scoop from the ablation and accumulation zones in 28 glaciers for DOC and TN concentration measurements. Surface ice samples were collected with a pre-cleaned ice axe at the ablation zones in 17 glaciers. Ice and snow samples were stored in well-sealed WhirlPak bags (WhirlPak®, Nasco, USA) and transported to the laboratory under frozen conditions.
Proglacial runoff samples were collected from 16 glaciers using 100 mL polycarbonate bottles pre-cleaned using 1 % HCl (Nalgene, USA). Totally eight sets of 24 h water samples were collected at 6 glaciers (i.e., MSTG, Tanggula Longxiazailongba (TGL), Qiangtang No. 1 (QT1), Qiangyong (QY), Tonkmadi (DKMD), and PL4). The conductivity and pH of proglacial runoff were measured in the field with a YSI EXO2 Water Quality Sonde. Water samples were transported in a dark container with ice. In the laboratory, samples were frozen at −20 ∘C until analyzed.
3.3 Laboratory measurements
The ice cores were decontaminated by removing the outer 1–2 cm annulus with a knife, then were cut into 5–10 cm long sections and transferred into containers in a −20 ∘C clean room. Both knife and container were pre-cleaned using 1 % HCl and rinsed with filter water. Snow, ice, and ice core samples were melted overnight and the meltwater was aliquoted into 20 mL glass bottles. The glass bottles were washed with 1 % HCl, rinsed with deionized water three times, and then combusted (450 ∘C for >3 h) before use.
Flow cytometry combined with the nucleic acid stain is a fast, accurate, quantitative, and reproducible technique for counting the number of bacteria (Hammes et al., 2008; Prest et al., 2013). The meltwater of snow, ice, and ice core samples were fixed with glutaraldehyde (final concentration: 1 %) and then stored at 4 ∘C, and were analyzed within 8 h after being stained with SYBR Green I (final concentration: ; Marie et al., 1997). SYBR Green I is the standard dye used to distinguish microorganisms from abiotic particles in various environments (Van Nevel et al., 2017; Mao et al., 2022). Stained samples were filtered using a cell strainer (pore size 420 µm) before being analyzed using flow cytometry to prevent clotting of the machine. The signal of microorganisms was differentiated from the inorganic particles based on both fluorescence intensity and size (Prest et al., 2013). Samples were processed on an EPICS ALTRA II flow cytometer (Beckman Coulter, USA) (Liu et al., 2016a). Duplicate samples were measured with a relative standard deviation lower than 10 %. Flow cytometry data were collected and analyzed with CytoWin 4.31 software.
The DOC and TN concentrations were measured using a TOC-Lcph (Shimadzu Corp., Japan) following standard methods (Greenberg et al., 1992). Concentrations of NO were measured using a Dionex ion Chromatograph System 2000 (Dionex Corp, USA) as previously described (Liu et al., 2016a).
4.1 Snowpit
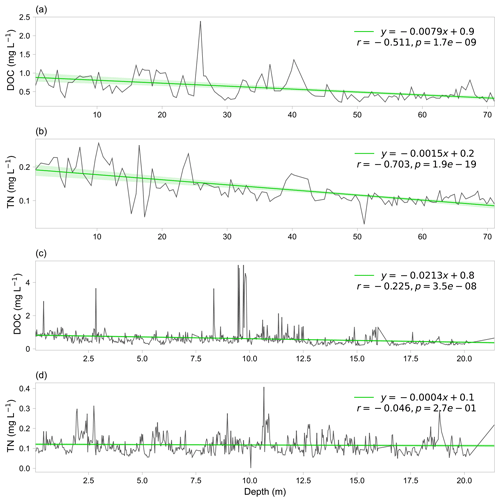
The microbial abundance in snow pits of the seven sampled glaciers ranged from 212 to 721 305 cells mL−1, and the mean microbial abundances were 2117, 8664, 218 305, 12 479, 14 442, 64 515, and 12 401 cells mL−1 for DD, GLDD, ZD, PL4, MDGR, Yala, and ERB, respectively. The range of these measurements is consistent with previous studies using the flow cytometer method (e.g., 3.7–25.0 × 104 cells mL−1 in the Kuytun 51 Glacier, Tianshan Mountains; Xiang et al., 2009; on the order of 103–105 cells mL−1 in the alpine snowpack; Lazzaro et al., 2015; Fillinger et al., 2021). Figure 3a shows the geospatial distribution of microbial abundance for the sampled snow pits. Generally, the microbial abundance is lower in the westerly dominated region than those in the monsoon dominated region. The DD glacier, located in the northeast of the TP, had the lowest microbial abundance (i.e., 2177 cells mL−1), while the ZD glacier, located in the south of the TP, had the highest abundance (i.e., 218 305 cells mL−1), which was 100 times higher than that in DD. Figure 4 shows the variation of microbial abundance with depth in each snow pit, but no consistent patterns were observed.
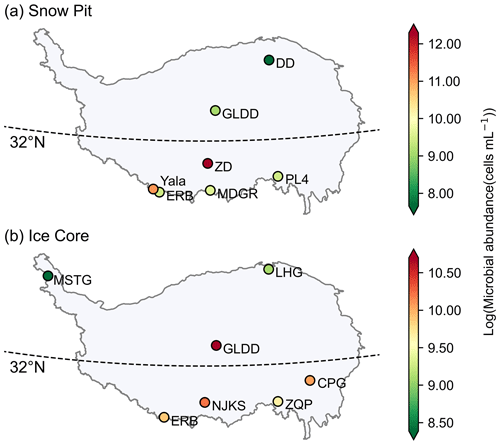
Figure 3The spatial distribution of log(microbial abundance) for sampled snow pits (a) and ice cores (b) averaged in each glacier.
4.2 Ice core
Microorganisms in glaciers are originated from atmospheric deposition, and it has been reported that microorganisms originating from the Saharan Desert have been found thousands of kilometers away in the Caribbean and European Alps (Kellogg and Griffin, 2006). The deposited microorganisms are subjected to a range of post-depositional environmental selection processes (Chen et al., 2022), until they are buried by snow and eventually frozen in the ice core. The microbial abundance in ice cores of the seven sampled glaciers varied substantially, ranging from 63 to 1 130 080 cells mL−1, and the mean microbial abundances were 4389, 8617, 44 318, 23 311, 15 648, 27 330, and 19 656 cells mL−1 for MSTG, LHG, GLDD, CPG, ZQP, NJKS, and ERB, respectively. These values generally are in agreement with previous studies using the flow cytometer method (e.g., on the order of 104–107 in the GISP2 Greenland ice core; Miteva et al., 2009; 6.53 × 103–2.89 × 105 cells mL−1 in the West Antarctic Ice Sheet Divide ice core; Santibáñez et al., 2016). The spatial distribution pattern of microbial abundance in each ice core is shown in Fig. 3b. Two ice cores in the north (i.e., MSTG and LHG) had a lower microbial abundance (i.e., 4389 and 8958 cells mL−1, respectively) than those in the south where the microbial abundance was at least 15 688 cells mL−1. Figure 4 shows the variation of microbial abundance along with depth in each ice core. The microbial abundance of ice cores in CPG and NJKS glaciers had decreasing trends with depth, while that in the MSTG glacier had an increasing trend. There were low-frequency fluctuations observed for the microbial abundance in ice cores of ERB, GLDD, and LHG glaciers, and there were mainly high-frequency fluctuations in ZQP glacier.
5.1 Ice core
Organic carbon and nitrogen in ice cores can be from allochthonous or autochthonous sources. It has been reported that the wet DOC deposition ranges from 47 to 330 mg C m−2 yr−1 (Yan et al., 2020) and the wet N deposition ranges from 44 to 155 mg N m−2 yr−1 on the TP (Liu et al., 2015). In addition, microbial carbon fixation has also been reported in glacier surface microbiome, and the average fixation rate in cryoconite holes of four glaciers on the TP is 1.77 µmol C m−2 d−1 (the yearly rate is approximately 3.3 mg C m−2 yr−1 assuming a growing season from May to September; Zhang et al., 2021), which is substantially lower than the atmospheric deposition rate. The microbial nitrogen fixation rate has not been quantified, but a study in the Arctic region has been reported as 0.04 mg N m−2 yr−1 (Telling et al., 2011), which is again orders of magnitude lower than the atmospheric deposition.
The DOC concentrations in ice core samples ranged from 0.005 to 5.05 mg L−1 with an average value of 0.54 ± 0.38 mg L−1. These values are larger than the englacial DOC concentrations in global mountain glaciers reported by Hood et al. (2015) (0.01–1.20 mg L−1, 0.29 ± 0.03 mg L−1 on average), which may be related to the higher aerosol concentration of TP (Spracklen et al., 2011). The TN concentrations ranged from 0.001 to 1.15 mg L−1 with an average value of 0.24 ± 0.16 mg L−1. Figure 6 shows the variation in DOC and TN concentrations across the vertical profiles in ice cores of the MSTG and ERB glaciers. Generally, there are decreasing trends of DOC and TN with depth, suggesting that atmospheric deposition has been increasing in recent years. There exist large inter-annual variations in the serial data with some large values occasionally, which may be related to historical large sand storms.
5.2 Surface ice, surface snow, and snow pit
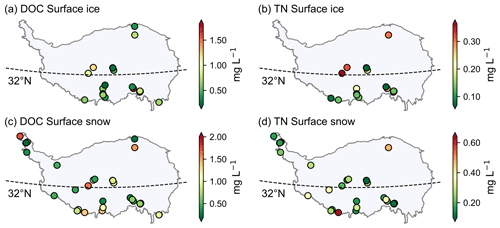
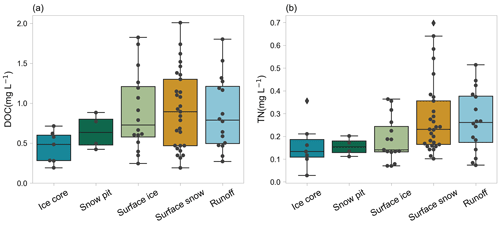
The DOC concentrations ranged from 0.08 to 9.0 mg L−1 (0.89 ± 1.05 mg L−1), from 0.12 to 11.65 mg L−1 (1.19 ± 1.78 mg L−1), and from 0.05 to 16.15 mg L−1 (0.72 ± 1.71 mg L−1) in surface ice, surface snow, and snow pits, respectively. These values are comparable to the DOC concentration of surface ice in four TP glaciers reported by Liu et al. (2016b) (i.e., 1.01 ± 0.22 mg L−1), and those in surface snow (mean values ranging from 0.16 to 1.17 mg L−1) and snow pits (mean values ranging from 0.21 to 0.81 mg L−1) in TP glaciers (Gao et al., 2020). The TN concentration ranged from 0.01 to 1.88 mg L−1 (0.19 ± 0.22 mg L−1), from 0.07 to 3.06 mg L−1 (0.34 ± 0.35 mg L−1), and from 0.02 to 0.84 mg L−1 (0.15 ± 0.15 mg L−1) in surface ice, surface snow, and snow pits, respectively. Figure 7 shows the spatial distribution of DOC and TN concentrations for surface snow and surface ice. For the DOC concentrations of surface snow, there is a decreasing trend from south to north in the monsoon dominated region (i.e., located to the south the 32∘ N). The DOC and TN concentrations in the westerly wind dominated and monsoon dominated regions were not significantly different (Mann–Whitney U test, p=0.95). The differences in TN concentration between the two regions were evident with higher values identified in the westerly wind dominated region, although the Mann–Whitney U test was not significant with a p value of 0.056.
5.3 Runoff
The DOC concentration ranged from 0.16 to 4.94 mg L−1 (0.79 ± 0.68 mg L−1) in the proglacial runoff, which is consistent with those observed worldwide (42 glaciers, 0.10–3.40 mg L−1; Li et al., 2018). The TN concentration ranged from 0.05 to 2.3 mg L−1 (0.29 ± 0.22 mg L−1). The runoff is neutral to alkaline with the pH value ranging from 7.3 to 12.4 (9.10 ± 0.88). Figure 8 shows the spatial distribution of DOC, TN, NO concentrations, and pH for the proglacial runoff on the TP. For the DOC concentration of runoff, there was a decreasing trend from west to east. There were significant differences in the concentrations of TN and NO in the runoff between the westerly wind and monsoon regions with p values both less than 0.01 (Mann–Whitney U test) and the values in the westerly wind dominated region were higher. The pH value was also larger in the westerly wind dominated region than in the monsoon dominated region, although the Mann–Whitney U test was not significant with a p value of 0.07.
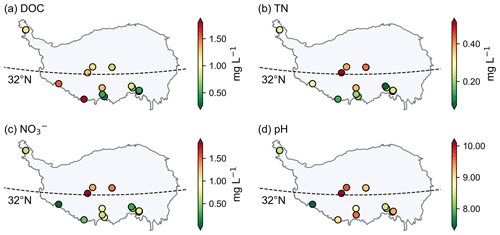
Figure 8The spatial distribution of DOC (a), TN (b), NO (c) concentrations, and pH (d) for proglacial runoff on the Tibetan Plateau.
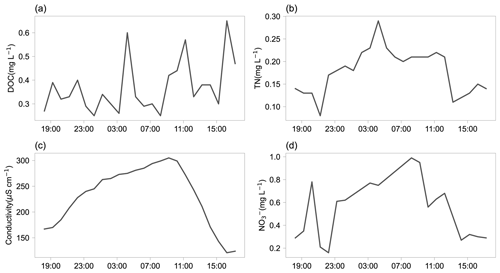
Figure 9The 24 h time series of DOC (a), TN (b), conductivity (c), and NO (d) in proglacial runoff of the Qiangyong glacier.
Figure 9 shows the diurnal variation of DOC, TN, NO, and conductivity in proglacial runoff of the Qiangyong glacier. No obvious pattern in the diurnal curve of DOC was observed, while there were obvious unimodal patterns for TN, NO, and conductivity. These observations are very helpful for biogeochemical studies at fine temporal resolutions.
5.4 Comparison among different habitats
The DOC and TN concentrations were compared among the various glacial habitats (ice core, snow pits, surface ice, surface snow, and proglacial runoff). The result showed that the DOC concentration in ice core was significantly lower than that in surface ice (Mann–Whitney–Wilcoxon test, P=0.02), while the differences of TN in ice core and surface ice were not significant (P=0.24). The DOC and TN concentrations were also lower in snow pits than in surface snow. The difference was not significant for DOC with a p value of 0.19 and was significant for TN with a p value of 0.03. The DOC and TN concentrations in proglacial runoff were similar to those in surface snow (Mann–Whitney U test, P=0.95 and 1.0 for DOC and TN, respectively).
The dataset of microbial abundance, DOC, and TN in the ice core, snow pit, surface ice, and snow from the Tibetan Plateau glaciers are accessible at the National Tibetan Plateau/Third Pole Environment Data Center (https://doi.org/10.11888/Cryos.tpdc.271841, Liu, 2021).
We constructed a dataset of microbial abundance, DOC, and TN for glaciers on the TP. The dataset comprises 5409 microbial abundance data from 12 glaciers and 2532 DOC and TN data from 38 glaciers. The sampled glaciers cover diverse geographic and climatic regions, which makes the dataset qualified for large-scale studies across the TP. This systematic dataset can provide important information on carbon and nitrogen cycles in glacial ecosystems. It can be used to evaluate the response of carbon and nitrogen cycling to global climate change, and to estimate the impact of glacier melting on downstream ecosystems such as glacier-fed streams and lakes. The time-series data of microbial abundance in ice cores can be used as an indicator of past climate change, and the spatial distribution of DOC and TN data can be used to estimate the storage and spatial distribution of glacier carbon and nitrogen, which are essential inputs for biogeochemical models of glacial ecosystems. Considering its broad spatial and temporal coverage, this dataset can serve as an important data source for forecasting the impact of warming on glacial carbon and nitrogen cycles at regional and even global scales.
The supplement related to this article is available online at: https://doi.org/10.5194/essd-14-2303-2022-supplement.
YL and JL designed the study and wrote the manuscript. PF and JL compiled and analyzed the dataset. YL, BG, MJ, PL, GM, BX, and SK performed field sampling and experimental measurement. All authors contributed to the writing and editing of this paper.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue “Extreme environment datasets for the three poles”. It is not associated with a conference.
We would like to thank Keshao Liu and Feng Wang for their great support in data checking and processing.
This research has been supported by the National Key Research and Development Program of China (grant no. 2019YFC1509103), the Second Tibetan Plateau Scientific Expedition and Research program (grant no. 2019QZKK0503), and the National Natural Science Foundation of China (grant nos. U21A20176 and 42171132).
This paper was edited by David Carlson and reviewed by Arwyn Edwards and one anonymous referee.
Anesio, A. M. and Laybourn-Parry, J.: Glaciers and ice sheets as a biome, Trends Ecol. Evol., 27, 219–225, https://doi.org/10.1016/j.tree.2011.09.012, 2012.
Anesio, A. M., Lutz, S., Chrismas, N. A. M., and Benning, L. G.: The microbiome of glaciers and ice sheets, npj Biofilms Microbiomes, 3, 10, https://doi.org/10.1038/s41522-017-0019-0, 2017.
Chen, Y., Liu, K., Liu, Y., Vick-Majors, T. J., Wang, F., and Ji, M.: Temporal variation of bacterial community and nutrients in Tibetan glacier snowpack, The Cryosphere, 16, 1265–1280, https://doi.org/10.5194/tc-16-1265-2022, 2022.
Dubnick, A., Wadham, J., Tranter, M., Sharp, M., Orwin, J., Barker, J., Bagshaw, E., and Fitzsimons, S.: Trickle or treat: The dynamics of nutrient export from polar glaciers, Hydrol. Process., 31, 1776–1789, https://doi.org/10.1002/hyp.11149, 2017.
Fell, S. C., Carrivick, J. L., Cauvy-Fraunié, S., Crespo-Pérez, V., Hood, E., Randall, K. C., Nicholass, K. J. M., Tiegs, S. D., Dumbrell, A. J., and Brown, L. E.: Fungal decomposition of river organic matter accelerated by decreasing glacier cover, Nat. Clim. Change, 11, 349–353, https://doi.org/10.1038/s41558-021-01004-x, 2021.
Fellman, J. B., Hood, E., Raymond, P. A., Hudson, J., Bozeman, M., and Arimitsu, M.: Evidence for the assimilation of ancient glacier organic carbon in a proglacial stream food web, Limnol. Oceanogr., 60, 1118–1128, https://doi.org/10.1002/lno.10088, 2015.
Fillinger, L., Hürkamp, K., Stumpp, C., Weber, N., Forster, D., Hausmann, B., Schultz, L., and Griebler, C.: Spatial and Annual Variation in Microbial Abundance, Community Composition, and Diversity Associated With Alpine Surface Snow, Front. Microbiol., 12, 781904, https://doi.org/10.3389/fmicb.2021.781904, 2021.
Gao, T., Kang, S., Zhang, Y., Sprenger, M., Wang, F., Du, W., Wang, X., and Wang, X.: Characterization, sources and transport of dissolved organic carbon and nitrogen from a glacier in the Central Asia, Sci. Total Environ., 725, 138346, https://doi.org/10.1016/j.scitotenv.2020.138346, 2020.
Greenberg, A. E., Clesceri, L. S., and Andrew, D. E.: Standard methods for the examination of water and wastewater, 18th edn., American Public Health Association, Washington, DC, USA, ISBN 0875532071, 1992.
Hammes, F., Berney, M., Wang, Y., Vital, M., Köster, O., and Egli, T.: Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes, Water Res., 42, 269–277, https://doi.org/10.1016/j.watres.2007.07.009, 2008.
Hodson, A., Anesio, A. M., Tranter, M., Fountain, A., Osborn, M., Priscu, J., Laybourn-Parry, J., and Sattler, B.: Glacial ecosystems, Ecol. Monogr., 78, 41–67, https://doi.org/10.1890/07-0187.1, 2008.
Hood, E., Fellman, J., Spencer, R. G. M., Hernes, P. J., Edwards, R., D'Amore, D., and Scott, D.: Glaciers as a source of ancient and labile organic matter to the marine environment, Nature, 462, 1044–1047, https://doi.org/10.1038/nature08580, 2009.
Hood, E., Battin, T. J., Fellman, J., O'Neel, S., and Spencer, R. G. M.: Storage and release of organic carbon from glaciers and ice sheets, Nat. Geosci., 8, 91–96, https://doi.org/10.1038/ngeo2331, 2015.
Hu, Z., Kang, S., Yan, F., Zhang, Y., Li, Y., Chen, P., Qin, X., Wang, K., Gao, S., and Li, C.: Dissolved organic carbon fractionation accelerates glacier-melting: A case study in the northern Tibetan Plateau, Sci. Total Environ., 627, 579–585, https://doi.org/10.1016/j.scitotenv.2018.01.265, 2018.
Immerzeel, W. W., van Beek, L. P. H., and Bierkens, M. F. P.: Climate Change Will Affect the Asian Water Towers, Science, 328, 1382, https://doi.org/10.1126/science.1183188, 2010.
Irvine-Fynn, T. D. L., Edwards, A., Stevens, I. T., Mitchell, A. C., Bunting, P., Box, J. E., Cameron, K. A., Cook, J. M., Naegeli, K., Rassner, S. M. E., Ryan, J. C., Stibal, M., Williamson, C. J., and Hubbard, A.: Storage and export of microbial biomass across the western Greenland Ice Sheet, Nat. Commun., 12, 3960, https://doi.org/10.1038/s41467-021-24040-9, 2021.
Kang, S., Zhang, Y., Chen, P., Guo, J., Zhang, Q., Cong, Z., Kaspari, S., Tripathee, L., Gao, T., Niu, H., Zhong, X., Chen, X., Hu, Z., Li, X., Li, Y., Neupane, B., Yan, F., Rupakheti, D., Gul, C., Zhang, W., Wu, G., Yang, L., Wang, Z., and Li, C.: Black carbon and organic carbon dataset over the Third Pole, Earth Syst. Sci. Data, 14, 683–707, https://doi.org/10.5194/essd-14-683-2022, 2022.
Kellogg, C. A. and Griffin, D. W.: Aerobiology and the global transport of desert dust, Trends Ecol. Evol., 21, 638–644, https://doi.org/10.1016/j.tree.2006.07.004, 2006.
Lazzaro, A., Wismer, A., Schneebeli, M., Erny, I., and Zeyer, J.: Microbial abundance and community structure in a melting alpine snowpack, Extremophiles, 19, 631–642, https://doi.org/10.1007/s00792-015-0744-3, 2015.
Li, X., Ding, Y., Xu, J., He, X., Han, T., Kang, S., Wu, Q., Mika, S., Yu, Z., and Li, Q.: Importance of Mountain Glaciers as a Source of Dissolved Organic Carbon, J. Geophys. Res.-Earth, 123, 2123–2134, https://doi.org/10.1029/2017JF004333, 2018.
Liu, K., Liu, Y., Hu, A., Wang, F., Zhang, Z., Yan, Q., Ji, M., and Vick-Majors, T. J.: Fate of glacier surface snow-originating bacteria in the glacier-fed hydrologic continuums, Environ. Microbiol., 23, 6450–6462, https://doi.org/10.1111/1462-2920.15788, 2021.
Liu, S., Yao, X., Guo, W., Xu, J., Shangguan, D., Wei., J., Bao., W., and Wu., L.: The contemporary glaciers in China based on the Second Chinese Glacier Inventory, Acta Geographica Sinica, 70, 3–16, 2015 (in Chinese).
Liu, Y.: Dataset of microbial abundance, dissolved organic carbon, and total nitrogen in Tibetan Plateau glaciers, National Tibetan Plateau Data Center [data set], https://doi.org/10.11888/Cryos.tpdc.271841, 2021.
Liu, Y., Priscu, J. C., Yao, T., Vick-Majors, T. J., Xu, B., Jiao, N., Santibáñez, P., Huang, S., Wang, N., Greenwood, M., Michaud, A. B., Kang, S., Wang, J., Gao, Q., and Yang, Y.: Bacterial responses to environmental change on the Tibetan Plateau over the past half century, Environ. Microbiol., 18, 1930–1941, https://doi.org/10.1111/1462-2920.13115, 2016a.
Liu, Y., Xu, J., Kang, S., Li, X., and Li, Y.: Storage of dissolved organic carbon in Chinese glaciers, J. Glaciol., 62, 402–406, https://doi.org/10.1017/jog.2016.47, 2016b.
Liu, Y. W., Xu-Ri, Wang, Y. S., Pan, Y. P., and Piao, S. L.: Wet deposition of atmospheric inorganic nitrogen at five remote sites in the Tibetan Plateau, Atmos. Chem. Phys., 15, 11683–11700, https://doi.org/10.5194/acp-15-11683-2015, 2015.
Mao, G., Ji, M., Xu, B., Liu, Y., and Jiao, N.: Variation of High and Low Nucleic Acid-Content Bacteria in Tibetan Ice Cores and Their Relationship to Black Carbon, Front. Microbiol., 13, 844432, https://doi.org/10.3389/fmicb.2022.844432, 2022.
Marie, D., Partensky, F., Jacquet, S., and Vaulot, D.: Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I, Appl. Environ. Microbiol., 63, 186–193, 1997.
Miteva, V., Teacher, C., Sowers, T., and Brenchley, J.: Comparison of the microbial diversity at different depths of the GISP2 Greenland ice core in relationship to deposition climates, Environ. Microbiol., 11, 640–656, https://doi.org/10.1111/j.1462-2920.2008.01835.x, 2009.
Prest, E. I., Hammes, F., Kötzsch, S., van Loosdrecht, M. C. M., and Vrouwenvelder, J. S.: Monitoring microbiological changes in drinking water systems using a fast and reproducible flow cytometric method, Water Res., 47, 7131–7142, https://doi.org/10.1016/j.watres.2013.07.051, 2013.
Santibáñez, P. A., Mcconnell, J. R., and Priscu, J. C.: A flow cytometric method to measure prokaryotic records in ice cores: an example from the West Antarctic Ice Sheet Divide drilling site, J. Glaciol., 62, 655–673, https://doi.org/10.1017/jog.2016.50, 2016.
Shi., Y. and Liu., S.: Estimation of the response of Chinese glaciers to global warming in the 21st century, Sci. Bull., 45, 434–438, 2000 (in Chinese).
Singer, G. A., Fasching, C., Wilhelm, L., Niggemann, J., Steier, P., Dittmar, T., and Battin, T. J.: Biogeochemically diverse organic matter in Alpine glaciers and its downstream fate, Nat. Geosci., 5, 710–714, https://doi.org/10.1038/ngeo1581, 2012.
Smith, H. J., Foster, R. A., McKnight, D. M., Lisle, J. T., Littmann, S., Kuypers, M. M. M., and Foreman, C. M.: Microbial formation of labile organic carbon in Antarctic glacial environments, Nat. Geosci., 10, 356–359, https://doi.org/10.1038/ngeo2925, 2017.
Spracklen, D. V., Jimenez, J. L., Carslaw, K. S., Worsnop, D. R., Evans, M. J., Mann, G. W., Zhang, Q., Canagaratna, M. R., Allan, J., Coe, H., McFiggans, G., Rap, A., and Forster, P.: Aerosol mass spectrometer constraint on the global secondary organic aerosol budget, Atmos. Chem. Phys., 11, 12109–12136, https://doi.org/10.5194/acp-11-12109-2011, 2011.
Telling, J., Anesio, A. M., Tranter, M., Irvine-Fynn, T., Hodson, A., Butler, C., and Wadham, J.: Nitrogen fixation on Arctic glaciers, Svalbard, J. Geophys. Res.-Biogeo., 116, G03039, https://doi.org/10.1029/2010JG001632, 2011.
Tian, L., Masson-Delmotte, V., Stievenard, M., Yao, T., and Jouzel, J.: Tibetan Plateau summer monsoon northward extent revealed by measurements of water stable isotopes, J. Geophys. Res.-Atmos., 106, 28081–28088, 2001.
Wadham, J. L., Hawkings, J., Telling, J., Chandler, D., Alcock, J., O'Donnell, E., Kaur, P., Bagshaw, E., Tranter, M., Tedstone, A., and Nienow, P.: Sources, cycling and export of nitrogen on the Greenland Ice Sheet, Biogeosciences, 13, 6339–6352, https://doi.org/10.5194/bg-13-6339-2016, 2016.
Van Nevel, S., Koetzsch, S., Proctor, C. R., Besmer, M. D., Prest, E. I., Vrouwenvelder, J. S., Knezev, A., Boon, N., and Hammes, F.: Flow cytometric bacterial cell counts challenge conventional heterotrophic plate counts for routine microbiological drinking water monitoring, Water Res., 113, 191–206, https://doi.org/10.1016/j.watres.2017.01.065, 2017.
Wang, Q., Yi, S., and Sun, W.: Continuous Estimates of Glacier Mass Balance in High Mountain Asia Based on ICESat-1,2 and GRACE/GRACE Follow-On Data, Geophys. Res. Lett., 48, e2020GL090954, https://doi.org/10.1029/2020GL090954, 2021.
Xiang, S.-R., Shang, T.-C., Chen, Y., Jing, Z.-F., and Yao, T.: Changes in diversity and biomass of bacteria along a shallow snow pit from Kuytun 51 Glacier, Tianshan Mountains, China, J. Geophys. Res.-Biogeo., 114, G04008, https://doi.org/10.1029/2008JG000864, 2009.
Yan, F., Wang, P., Kang, S., Chen, P., Hu, Z., Han, X., Sillanpää, M., and Li, C.: High particulate carbon deposition in Lhasa – a typical city in the Himalayan–Tibetan Plateau due to local contributions, Chemosphere, 247, 125843, https://doi.org/10.1016/j.chemosphere.2020.125843, 2020.
Yao, T., Xiang, S., Zhang, X., Wang, N., and Wang, Y.: Microorganisms in the Malan ice core and their relation to climatic and environmental changes, Global Biogeochem. Cy., 20, GB1004, https://doi.org/10.1029/2004GB002424, 2006.
Yao, T., Thompson, L., Yang, W., Yu, W., Gao, Y., Guo, X., Yang, X., Duan, K., Zhao, H., Xu, B., Pu, J., Lu, A., Xiang, Y., Kattel, D. B., and Joswiak, D.: Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings, Nat. Clim. Change, 2, 663–667, https://doi.org/10.1038/nclimate1580, 2012.
Yao, T., Xue, Y., Chen, D., Chen, F., Thompson, L., Cui, P., Koike, T., Lau, W. K.-M., Lettenmaier, D., and Mosbrugger, V.: Recent third pole's rapid warming accompanies cryospheric melt and water cycle intensification and interactions between monsoon and environment: Multidisciplinary approach with observations, modeling, and analysis, B. Am. Meteorol. Soc., 100, 423–444, https://doi.org/10.1175/BAMS-D-17-0057.1, 2019.
Zhang, S. H., Hou, S. G., Yang, G. L., and Wang, J. H.: Bacterial community in the East Rongbuk Glacier, Mt. Qomolangma (Everest) by culture and culture-independent methods, Microbiol. Res., 165, 336–345, https://doi.org/10.1016/j.micres.2009.08.002, 2010.
Zhang, X. F., Yao, T. D., Tian, L. D., Xu, S. J., and An, L. Z.: Phylogenetic and Physiological Diversity of Bacteria Isolated from Puruogangri Ice Core, Microb. Ecol., 55, 476–488, https://doi.org/10.1007/s00248-007-9293-3, 2008.
Zhang, Y., Kang, S., Wei, D., Luo, X., Wang, Z., and Gao, T.: Sink or source? Methane and carbon dioxide emissions from cryoconite holes, subglacial sediments, and proglacial river runoff during intensive glacier melting on the Tibetan Plateau, Fundam. Res., 1, 232–239, https://doi.org/10.1016/j.fmre.2021.04.005, 2021.
- Abstract
- Introduction
- Study area
- Sample distribution and laboratory measurements
- Data description of microbial abundance
- Data description of DOC and TN
- Data availability
- Conclusions
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- Study area
- Sample distribution and laboratory measurements
- Data description of microbial abundance
- Data description of DOC and TN
- Data availability
- Conclusions
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement