the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Soil and tree stem xylem water isotope data from two pan-European sampling campaigns
Josie Geris
Ilja van Meerveld
Daniele Penna
Youri Rothfuss
Matteo Verdone
Pertti Ala-Aho
Matyas Arvai
Alise Babre
Philippe Balandier
Fabian Bernhard
Lukrecija Butorac
Simon D. Carrière
Natalie C. Ceperley
Zuosinan Chen
Alicia Correa
Haoyu Diao
David Dubbert
Maren Dubbert
Fabio Ercoli
Marius G. Floriancic
Alligin Ghazoul
Teresa E. Gimeno
Damien Gounelle
Frank Hagedorn
Christophe Hissler
Frédéric Huneau
Alberto Iraheta
Tamara Jakovljević
Nerantzis Kazakis
Zoltan Kern
Laura Kinzinger
Karl Knaebel
Johannes Kobler
Jiri Kocum
Charlotte Koeber
Gerbrand Koren
Angelika Kübert
Dawid Kupka
Samuel Le Gall
Aleksi Lehtonen
Thomas Leydier
Philippe Malagoli
Francesca Sofia Manca di Villahermosa
Chiara Marchina
Núria Martínez-Carreras
Nicolas Martin-StPaul
Hannu Marttila
Aline Meyer Oliveira
Gael Monvoisin
Natalie Orlowski
Kadi Palmik-Das
Aurel Persoiu
Andrei Popa
Egor Prikaziuk
Cécile Quantin
Katja T. Rinne-Garmston
Clara Rohde
Martin Sanda
Matthias Saurer
Daniel Schulz
Michael P. Stockinger
Christine Stumpp
Jean-Stéphane Vénisse
Lukas Vlcek
Stylianos Voudouris
Björn Weeser
Mark E. Wilkinson
Giulia Zuecco
Katrin Meusburger
The stable isotope ratios of hydrogen (δ2H) and oxygen (δ18O) are useful for studying ecohydrological dynamics in forests. However, most isotope-based eco-hydrological studies are limited to single sites, resulting in a lack of large-scale isotope data for understanding tree water uptake. Here, we provide a first systematic isotope dataset for soil and stem xylem water collected during two pan-European sampling campaigns at 40 beech (Fagus sylvatica), spruce (Picea abies), or mixed beech-spruce forest sites in spring and summer 2023 (https://doi.org/10.16904/envidat.542, Lehmann et al., 2024). The dataset is complemented by additional site-, soil-, and tree-specific metadata. The samples and metadata were collected by different researchers across Europe following a standardized protocol. Soil samples were taken at up to 5 depths (ranging from 0 to 90 cm) and stem xylem samples from the trunks of three beech and/or spruce trees per site. All samples were sent to a single laboratory, where all analytical work was conducted. Water was extracted using cryogenic vacuum distillation and analyzed with an isotope laser spectrometer. Additionally, a subset of the samples was analyzed with an isotope ratio mass spectrometer. Data quality checks revealed a high mean total extraction efficiency, mean water amount (>1 mL), accuracy, and precision. The isotopic signature of soil and stem xylem water varied as a function of the geographic origin and changed from spring to summer across all sites. While δ2H and δ18O were strongly correlated, the soil water data plotted closer to the Global Meteoric Water Line (GMWL) than the stem xylem water. Specifically, the δ2H values of the xylem water were more enriched than those of the soil water, leading to a systematic deviation from the GMWL. Isotopic enrichment of the stem xylem water at mixed forest sites was larger for spruce trees than for beech trees. This dataset is particularly useful for large-scale studies on plant water use, ecohydrological model testing, and isotope mapping across Europe.
- Article
(4074 KB) - Full-text XML
-
Supplement
(631 KB) - BibTeX
- EndNote
Understanding how tree water uptake from soils varies with species, site characteristics, time, and across climate zones is essential to assess forest resistance and resilience to climate change; particularly the response of forests to the increasing frequency and intensity of droughts (Lindner et al., 2010; Spinoni et al., 2014; Büntgen et al., 2021). Despite some uncertainties, the stable isotope ratios of hydrogen (δ2H) and oxygen (δ18O) in water extracted from soil and plants allow for the estimation of the sources of water that are used by the plants and to quantify the relative contributions of different water sources to plant water use (Rothfuss and Javaux, 2017; Beyer and Penna, 2021). Estimates of water uptake patterns based on isotope data assume that roots do not discriminate against the heavier stable isotopes during water uptake (Poca et al., 2019). Additionally, it is assumed that: (i) the sampling design captures all end-members with a proper representation of the spatiotemporal variability of their isotopic composition, (ii) the water extracted from the plant xylem is a mixture of the different water sources taken up from the soil profile without isotopic alteration (e.g., due to stem evaporation, see Ellsworth and Sternberg, 2015), and (iii) soil and xylem samples are collected, transported, stored, and extracted in a manner that avoids isotope fractionation (Ceperley et al., 2024). Although these assumptions are not always met, the method can either independently or in combination with other measurements (e.g., in combination with assessment of physiological or hydraulic traits) be used to effectively determine plant responses to both short- and long-term droughts. Isotope-based analyses in forest ecosystems have, for example, been used to determine the changes in root water uptake depths of trees in response to drought (Brinkmann et al., 2018; Gessler et al., 2022), whether trees use summer or winter precipitation (Allen et al., 2019; Floriancic et al., 2024a) and whether they use soil water, groundwater, or streamwater (Bowling et al., 2017; Engel et al., 2022), or to assess competitive or complementary water use strategies (Penna et al., 2020; Kinzinger et al., 2024). The method is now affordable enough for practical applications beyond the field of isotope ecohydrology (Penna et al., 2018).
However, systematic datasets at large scales, i.e., spanning continents or multiple countries, are lacking. This hampers our understanding of how water uptake strategies for the same tree species vary across space and time (Beyer and Penna, 2021; Orlowski et al., 2023; Dubbert and Werner, 2019; Bachofen et al., 2024). There are established networks for the observation of isotopes in freshwater systems, such as precipitation by the International Atomic Energy Agency (IAEA) Global Network of Isotopes in Precipitation (GNIP), which currently contains data for 300 active sites in 93 countries (Terzer-Wassmuth et al., 2023). The Global Network of Isotopes in Rivers (GNIR) contains data from 750 sites in 35 countries (Halder et al., 2015). Both networks provide valuable input data for modeling of local to regional climate or surface-atmosphere water interactions with process-based (e.g., CLM, Wong et al., 2017; ISOLSM, Cai et al., 2015; ECHAM5-JSBACH, Haese et al., 2013) or statistical models (e.g., Isoscapes; Bowen, 2010; Terzer et al., 2013; Allen et al., 2018; Koeniger et al., 2022), and time series analyses (Nelson et al., 2021; Erdélyi et al., 2023; Reckerth et al., 2017). They have furthermore helped to assess water flow pathways and the fraction of young water in streamflow (Von Freyberg et al., 2018; Floriancic et al., 2024b). The Moisture Isotopes in Biosphere and Atmosphere (MIBA) network, initiated by the IAEA in 2003–2004, is a rare example of an international network to survey the isotopic composition of water across different ecosystem compartments (i.e., soil, plant stems and leaves, and atmospheric vapor). However, despite the global distribution of sites at the time of the establishment and a local application in Australia (Twining et al., 2006), the network is currently inactive.
Building on the idea of the MIBA and the proven usefulness of national large-scale sampling campaigns to determine regional differences in tree water uptake (Allen et al., 2019), the COST Action “WATer isotopeS in the critical zONe: from groundwater recharge to plant transpiration WATSON” (CA19120) organized two sampling campaigns across Europe in 2023. The effort took advantage of the European network of researchers to establish a unique systematic water isotope dataset and corresponding metadata. More specifically, the goal of the sampling campaigns was to obtain soil and stem xylem water isotope data of two tree species, namely beech (Fagus sylvatica L.) and spruce (Picea abies (L.) H. Karst) across a large climate gradient for the spring (25 May to 16 June) and summer (17 August to 18 September) of 2023. The two time points were selected to compare tree water uptake patterns under different soil moisture conditions (i.e., expected lower soil moisture in summer). The two species were selected because of their wide geographical distribution across Europe (Fig. 1), their ecological and economical relevance, and the expected differences in water uptake depth (Allen et al., 2019; Brinkmann et al., 2018; Goldsmith et al., 2019) because beech trees typically have a deeper rooting system than spruce trees.
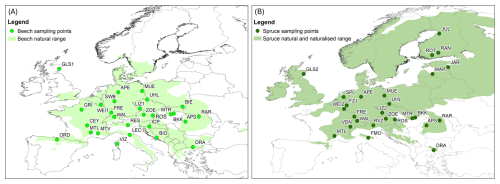
Figure 1Maps showing the sampling sites (circles) for beech (A) and spruce (B) trees and their natural and naturalised ranges across Europe (shaded areas; data from Caudullo et al., 2017).
During the sampling campaigns, a total of 381 soil and 311 stem (i.e., trunk) xylem samples were taken from 40 sites in 18 countries, following a standardized protocol. The water of these samples was cryogenically extracted and analyzed for its isotopic composition in a single laboratory. The simultaneous collection of soil and stem xylem samples across all sites, combined with the centralized processing of the samples, results in a unique dataset. Using one laboratory prevents inconsistencies that might arise from varying sample handling and analysis methods, which can lead to isotopic offsets (Orlowski et al., 2016, 2018). The isotope dataset is accompanied by site-, soil-, and tree-specific metadata for each site. This includes geographic details, information on soil type, texture and maximum depth, details on forest stands, tree diameter and height, sampling information, as well as data on canopy cover/gap fractions as indicators for stand density and tree health and crown defoliation (Bussotti et al., 2024). Together, the metadata and isotope data provide a strong foundation for future research on tree water use, model testing, and isotope mapping. This manuscript outlines the sample collection process, cryogenic water extraction and isotope analysis methods, and details on the dataset organization and metadata. Finally, we give an overview of the data and discuss potential applications. The full dataset is freely available from the Envidat repository (Lehmann et al., 2024).
2.1 Organization of the WATSON pan-European sampling campaigns
During the initial phase (spring 2023), the members of the WATSON community (∼200 members at that time) were contacted to assess their interest in participating in a coordinated sampling campaign. Based on the large interest, a core team was formed. The core team asked researchers from a similar region to form one team and decide on a single sampling location to keep the laboratory and analytical work manageable, while still obtaining samples from a broad geographic region. The core team wrote detailed instructions to ensure a consistent sampling procedure at all sites. The instructions provided detailed standardized protocols for collecting the soil samples and stem xylem samples from trunks, including specifications for sampling depths, core dimensions, and the maximum number of samples. The protocols also covered short-term sample storage and shipment to the Swiss Federal Institute for Forest, Snow, and Landscape Research in Birmensdorf, Switzerland (WSL Birmensdorf), where all cryogenic water extractions and isotopic analyses were performed. In addition, participants were given instructions on how to take pictures for canopy cover analysis and the list of required metadata (e.g., geograph location, soil properties, tree diameter and height). The instructions were emailed to all interested contributors prior to the first sampling campaign in spring 2023 (Sect. S1 in the Supplement). For the second campaign in summer 2023, the sampling protocol was slightly updated for clarity (i.e., addition of the weather conditions on the sampling day, bark removal during stem xylem sampling, a reminder to avoid sampling the heartwood, labelling of exetainers, taking photos) and was again sent to all interested contributors by email (Sect. S2 in the Supplement). In addition, we held an online meeting between the two sampling campaigns to provide feedback to the participants, clarify any field issues, and answer questions.
2.2 Description of the sampling sites
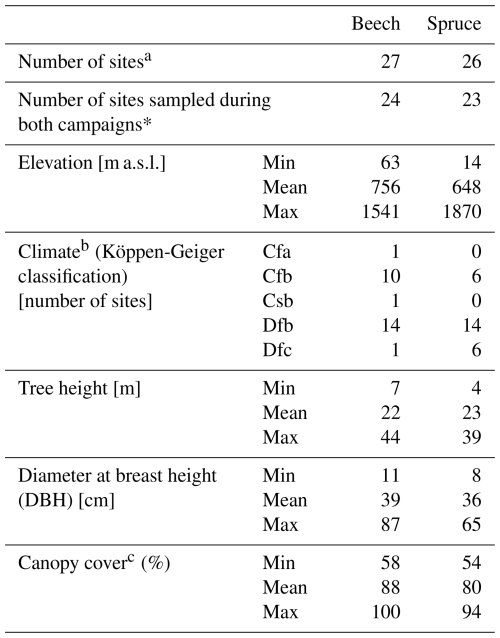
Samples were taken from different mono-specific forest sites with beech trees (Fagus sylvatica; 14 sites), spruce trees (Picea abies; 13 sites), or mixed forest sites with both tree species (13 sites). Of the 40 sites located in 18 European countries (Fig. 1; Table 1), 36 were sampled in the spring and 39 in the summer. For 35 of the 40 sites, samples were collected during both campaigns. At three of the sampling sites, beech (LIZ1, GLS1, WEI1) and spruce (LIZ2, GLS2, WEI2) stands were found close to each other (i.e., the sampling sites share the same coordinates).
Table 1Summary statistics for the two sampling campaigns across 18 European countries.
a these numbers include the 13 sites with both species.
b Köppen-Geiger classification based on Beck et al. (2023).
c based on the average value for all photos for each sampling site.
Although there was a good cover of sites across central Europe for both species, most north-eastern sites were sampled for spruce only; the sampled beech trees extended more to south-western Europe. The sampling sites correspond to the natural and naturalised ranges of the tree species across Europe (Fig. 1) and cover a range of temperate (Köppen-Geiger Cfa, Cfb, Csb) and cold (Köppen-Geiger Dfb, Dfc) climates. The sampling sites differed in elevation (14–1870 m a.s.l.; Table 1). The sampling sites were evenly distributed across different slope categories (i.e., flat, gentle, and steep). Most sites were located on Cambisols or Leptosols; with just one Histosol (i.e., peat at the ROT site in Finland). The maximum soil depth varied between 0.3 and >1 m. For half of the sites was the maximum soil depth >0.6 m.
Canopy cover was estimated for 30 of the 40 sampling sites from non-hemispherical photographs taken systematically at varying distances from the stem with a smartphone camera (Sect. S3 in the Supplement). Most of the pictures were taken during the spring campaign, however, for some sites, pictures were taken during the summer campaign or both campaigns. For the sites for which canopy cover could be determined, it was generally higher for the beech trees than the spruce trees (Table 1).
2.3 Sampling, transport, and storage of stem xylem and soil samples
At each sampling site, three beech (Fagus sylvatica) and/or three spruce (Picea abies) trees were selected based on their representativeness for the stand. The selected spruce and beech trees ranged in size but were similar in mean height (22–23 m) and diameter at breast height (36–39 cm, Table 1). Stem xylem samples were taken from the trunk of each selected tree at breast height using a 0.5 cm increment borer. Thus, in this study, “stem” refers specifically to the trunk of the tree, excluding branches and other aboveground components. The same three trees were sampled during both campaigns at each site, except at the beech site GRI, where different trees were sampled in spring and summer, and at the beech site MTV, where samples were taken from six trees. This resulted in a total of 311 stem xylem samples. Each stem xylem sample (one per selected tree) consisted of two to three generally intact wood cores, with an average length of 5.5±1.5 cm for beech and 4.8±1.6 cm for spruce (mean ± SD). The outer and inner bark of the wood cores were removed from the cores, yet, bark residue was observed in 40 % of all stem xylem samples after cryogenic water extraction. The wood cores mainly reflect sapwood as participants were instructed to avoid sampling the heartwood because there are indications of isotopic differences between sapwood and heartwood (Fabiani et al., 2022). However, we cannot fully rule out the presence of heartwood in some samples as visual determination of the heartwood after water extraction was not possible. A heartwood correction based on mean wood core length and tree diameter could be developed. Such an adjustment may be particularly important for samples from smaller spruce trees, which are more likely to have a limited sapwood depth (Peters et al., 2019).
In addition to the stem xylem samples, soil samples were taken with a manual soil auger at a location between the selected trees. The samples were taken from a single soil core at three to five depths, typically at 10 cm intervals (0–10, 10–20, 20–30, 50–60, and 80–90 cm below the surface). In some cases, other depths were sampled, or the sampling interval was 20 cm. The number of soil samples and the depth of the deepest soil sample depended on the soil properties (e.g., rocky soils) and the maximum soil depth at the sampling location. The litter was removed before taking the 0–10 cm soil sample. At some sites and during certain campaigns, soil samples were also taken from two to three additional nearby locations (up to four in total), resulting in a varying number of samples and sampling depths. For a few sites with both species (i.e., DRA, FRE, UHL, ZOE), soil cores were taken separately for beech, spruce, and both species. In total 381 soil samples were taken.
Stem xylem and soil samples were transferred into 12 mL gas-tight glass vials (“Exetainers”, Labco, Lampeter, UK). For the soil samples, exetainers were filled for 50 %–80 % of their volume with soil. Some soil and stem xylem samples (13 % of all 692 samples) were stored in other types of gas-tight plastic or glass vials. Most samples were taken midday on dry and sunny days. Samples were handled as quickly as possible to avoid evaporative fractionation. Back in the laboratory, all samples were stored in a refrigerator to avoid moisture loss to evaporation and subsequent isotope fractionation (as well as to reduce microbial growth and the decomposition of the organic material) until transportation. All samples were then shipped without cooling and arrived within four weeks after the final day of each sampling campaign at the laboratory at WSL Birmensdorf in Switzerland, where they were kept at −20 °C until cryogenic water extraction.
2.4 Cryogenic vacuum water extraction
Water was extracted from all 692 samples at WSL Birmensdorf using a cryogenic vacuum distillation method as described in Diao et al. (2022). In brief, the exetainers with the samples were taken from the freezer and, for the soil samples, fitted with polypropylene fiber filters (Nozzle protection filter, Socorex Isba SA, Ecublens, Switzerland) to prevent particles from being drawn into the extraction line. Samples originally stored in other types of vials (N=90) were transferred to exetainers that fit the cryogenic vacuum distillation system. Samples were then heated to 80 °C in a water bath, while the extraction line was kept under a vacuum of <5 Pa (BS2212, Brook Crompton Ltd, Doncaster, UK). The extracted water was trapped in U-shaped glass tubes, kept in liquid nitrogen. After a minimum of 2 h, water extraction was stopped and atmospheric pressure was established in the extraction line by passing dry nitrogen gas through it. Then, the U-shaped tubes were removed, the ends of the tubes were closed with rubber plugs and the water samples were thawed at room temperature. Depending on the extracted water amount, the water was pipetted to 350 µL or 2 mL glass vials (Infochroma AG, Goldau, Switzerland) and kept frozen at −20 °C until isotope analysis. A few samples that appeared turbid after extraction were filtered with 0.45 µm nylon syringe filters (Infochroma AG).
We determined the sample weight before water extraction (“fw”), after water extraction (“dw1”), and after drying at 105 °C for 24 h (dw2) to estimate the absolute water amount (“awa”), the total extraction efficiency (“tef”), and the gravimetric water content (gwc) for each sample (for equations, see Table 3). The sample weights (i.e., “fw”, “dw1”, “dw2”) were corrected for the weight of the exetainer (“exe_weight”, Tables 3 and S1 in the Supplement). The latter was based on the mean weight of approximately thirty exetainers for 8 different types (“exe_type”) based on different combinations of glass vial shapes, caps with or without a rubber seal, and the presence of a sticky label (Tables 3 and S1). The average weight of the exetainers was 13.0±0.2 g (SD).
Across all soil and stem xylem samples (Fig. 2A), the extracted amount of water (“awa”) averaged around 1.4 mL and was well above the critical threshold for extracted water volume of 0.6 mL for the vast majority of samples (Diao et al., 2022). The average value for the total extraction efficiency (“tef”) was 100.6 % (Fig. 2B) and was for most samples (N=543) within the optimal range (Ceperley et al., 2024). The gravimetric water content (“gwc”) varied among sample types and averaged 41 % for soil, 61 % for beech xylem, and 84 % for spruce xylem samples (Fig. 2C). The very high soil gwc values (>200 %) were all obtained for samples from the ROT site and reflect the high organic matter content (i.e., peat soil) for this site. Note that variations in “awa”, “tef”, and “gwc”, as well as “tef” values >100 %, may partly be due to uncertainties arising from the exetainer weights (“exe_weight”; Table 3), reflecting an average value rather than the actual weight of each exetainer.
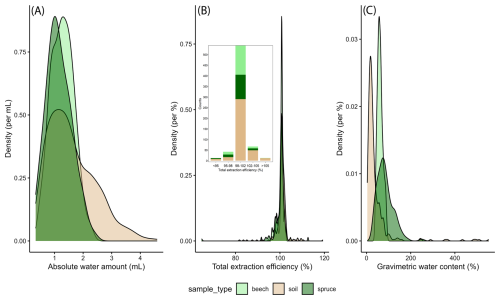
Figure 2Density plots for (A) the extracted absolute water amounts (awa), (B) the total extraction efficiency (tef), and (C) the gravimetric water content (gwc) for stem xylem (beech and spruce) and soil for all samples analysed (i.e., from all sites, depths and sampling campaigns). The inset in figure (B) shows the sample count for different types of samples across five different tef classes.
2.5 Isotope analysis with laser spectrometer and IRMS
The stable isotope ratios of hydrogen (δ2H) and oxygen (δ18O) of the cryogenically extracted water were measured at WSL Birmensdorf using a laser cavity ring-down spectrometer (L2140-i, Picarro Inc., Santa Clara, USA) connected to a micro-combustion module (MCM) to eliminate artefacts caused by co-extracted organic compounds (Martín-Gómez et al., 2015). Each sample was injected eight times and the average of the final five injections was taken to minimize memory effects (Penna et al., 2012). Samples were calibrated with four reference isotope standards spanning from −10.5 ‰ to −120.2 ‰ for δ2H and from −3.0 ‰ to −16.1 ‰ for δ18O (LGR; Envitec NV, Lessines, Belgium) and normalized to the international Vienna Standard Mean Ocean Water (VSMOW-2) scale. The maximum deviation (i.e., accuracy) of an interspersed in-house laboratory standard (analysed every ∼25 samples, δ2H: −84.9 ‰, δ18O: −9.6 ‰) from the expected value was ≤0.5 ‰ for δ2H and ≤0.2 ‰ for δ18O. The standard deviation (SD) of the repeated measurements of the laboratory standards (i.e., precision) was ≤0.6 ‰ for δ2H and ≤0.1 ‰ for δ18O.
To check for spectral interferences with plant-produced volatile organic compounds during the isotope analysis with the laser spectrometer, a subset of 83 samples were also analyzed using a thermal combustion/elemental analyzer (TC/EA) coupled to a DeltaPlus XP isotope ratio mass spectrometer (IRMS, Finnigan MAT, Bremen, Germany), with a typical precision of 1.0 ‰ for δ2H and 0.2 ‰ for δ18O. This subset contained samples from both sampling campaigns, all sample types (soils from different depths and stem xylem from both tree species), and a range of geographic locations and isotope values. The IRMS data were highly correlated with the data of the laser spectrometer (Fig. 3A and B). Most of the data were within the range of ±1 SD but showed a positive offset for both δ2H and δ18O (Fig. 3C). The δ2H and δ18O offsets between the two types of analysis had mean values around 0.7 ‰ and 0.3 ‰ across all samples (Fig. 3C), respectively. These mean offsets represent the average of the differences between the two methods, accounting for both positive and negative values. The SD of these offsets were 1.4 ‰ for δ2H and 0.5 ‰ for δ18O, indicating the variability around the mean offsets, not zero. Additionally, paired t-tests showed that the isotopic offsets in stem xylem samples between the two analytical methods depended on species (P<0.05), with larger offsets observed in spruce (mean δ2H=1.1 ‰, δ18O=0.7 ‰) than in beech (mean δ2H=0.7 ‰, δ18O=0.4 ‰). For soil samples, we observed a significant instrumental effect only for δ2H (mean difference=0.6 ‰).
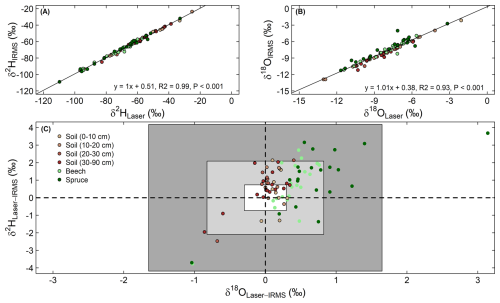
Figure 3Linear relationships between the δ2H (A) and δ18O (B) for the water samples analyzed using a laser spectrometer (Laser) and an isotope ratio mass spectrometer (IRMS). Panel (C) displays a biplot of the isotopic offsets in δ18O and δ2H for the two instruments. The small white box in the middle of C represents the mean δ2H and δ18O offsets across stem xylem and soil samples, while the light grey and dark grey boxes denote ± one and two standard deviations of the offsets, respectively.
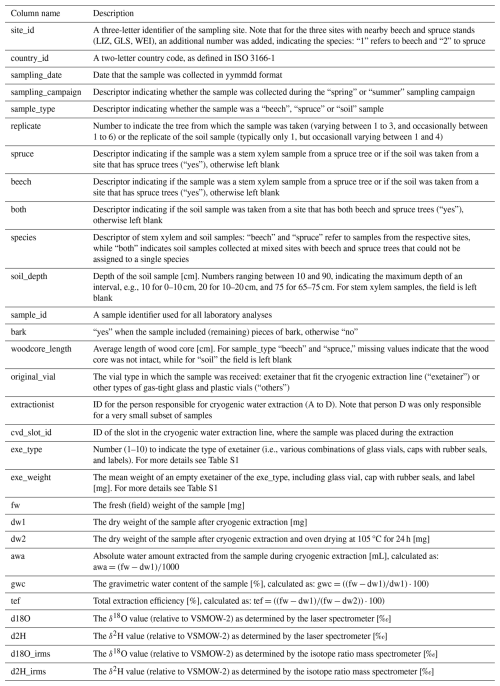
The dataset consists of three comma-separated files (.csv) and one zip file (.zip) with photos of the canopy at the sampling sites. All .csv files are encoded in UTF-8 and use commas as delimiters. The first datafile (“WATSON_Metadata.csv”) contains all the metadata about the sampling sites including site-, soil- and tree-specific information (Table 2). The second file (“WATSON_Isotopedata.csv”) contains the information about sample weights, cryogenic water extraction and the actual hydrogen and oxygen isotope data (Table 3). The third file (“WATSON_Canopydata.csv”) contains the information on the canopy cover (Table 4). The photos on which the canopy cover data are based are stored in the “WATSON_Canopy_Pictures.zip” file. Datasets can be linked by the site_id, a three-letter identifier representing each sampling site.
Table 2Description of the columns in the “WATSON_Metadata.csv” file containing all the meta-information about the sampling sites [and units].
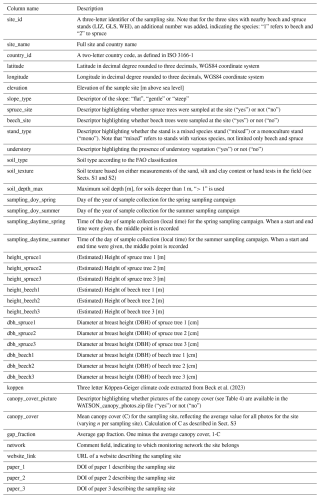
Table 3Description of the columns in the “WATSON_Isotopedata.csv” file containing all the isotope data and additional information about the extraction [and units].
4.1 Isotopic variation for the spring and summer sampling campaigns
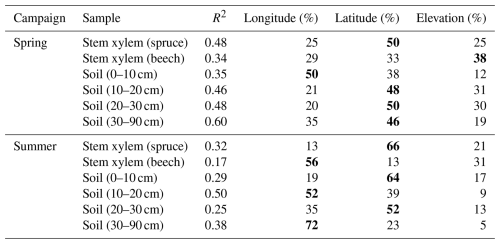
The isotopic composition of the soil and the stem xylem water samples varied spatially (Fig. 4). As expected, the samples were more depleted in heavy isotopes at sites located further north and inland. Multiple linear regression analysis showed that latitude, longitude, and elevation were all important variables to explain the observed spatial variation in the isotopic composition of soil and stem xylem water (Table 5). Among the three geographic variables, longitude and latitude explained most of the variance for seven of the eight cases shown in Table 5. Since the total variance explained by latitude, longitude, and elevation was relatively low (R2=0.17–0.60), other factors likely contributed to the variation in the isotopic composition of the samples. In combination with the gravimetric water content of the soil as a qualitative indicator of soil wetness (i.e., “gwc”; Table 3), gridded climate data, and precipitation isotope data (e.g., Nelson et al., 2021), the data could be useful for new soil and stem xylem water isoscape models and be used as complimentary data in hydrological studies.
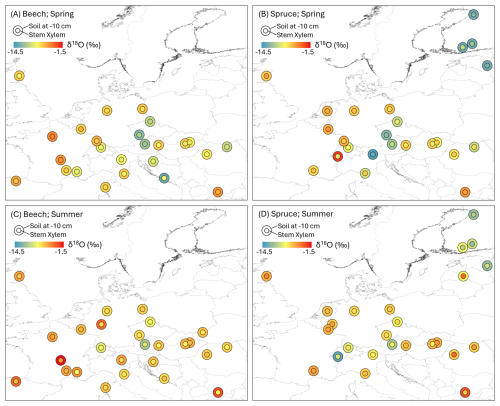
Figure 4Map showing the δ18O values for stem xylem water (inner circle) and soil water at 0–10 cm (outer circle) for the spring (A, B) and summer (C, D) sampling campaigns for the beech (A, C) and spruce (B, D) sites. For some sites, the isotopic composition of the stem xylem samples was similar to that of the soil at 0–10 cm depth (both circles have the same color); for others, the differences were large (i.e., the color of the inner and outer circle differs) indicating water uptake from a different (e.g., deeper) water source.
Table 5Percentage of variance in δ18O values explained by latitude, longitude, and elevation, as determined by multiple linear regression analysis. Values in bold indicate the highest relative contribution of a geographical parameter to the total variance for each sample type for each campaign (Spring/Summer). R2 reflects the total variance explained by latitude, longitude, and elevation. All linear models were statistically significant (P<0.001).
The isotopic composition of the soil and stem xylem water samples also varied between the two sampling campaigns (Figs. 4 and 5). δ18O values were higher in summer compared to those of the spring for stem xylem water of both species and for soil water at 0–10 cm, 10–20 cm and 20–30 cm (unpaired t-test, P<0.05). The δ18O values of soil water at depths of 30–90 cm did not differ seasonally (unpaired t-test, P>0.05; Fig. 5). For the site-level mean δ18O values of stem xylem water (i.e., the average δ18O value for all trees at a site), the median seasonal difference (summer-spring) was 0.6 ‰ across all beech sites (ranging from −1.9 ‰ to 2.9‰) and 0.8 ‰ across all spruce sites (ranging from −1.4‰ to 4.8‰). For site-level mean δ18O values of soil water (i.e., the average δ18O value for a soil of a specific depth range; in most cases only a single value), the median seasonal difference was larger and/or more variable, e.g., 1.3 ‰ at 0–10 cm depth (ranging from −10.8‰ to 6.1‰) and 0.6 ‰ at 30–90 cm depth (ranging from −3.3‰ to 9.6‰). Comparisons across all soil depths shows that in spring, site-level mean δ18O values of soil water at 30–90 cm depth were lower (i.e., more negative) compared to those at 0–10 cm (unpaired t-test, P<0.05) but not to those at 10–20 cm or 20–30 cm (unpaired t-test, both P>0.05). In contrast, in summer δ18O values at 30–90 cm depth were lower than those at 0–10, 10–20 and 20–30 cm (unpaired t-test, P<0.05). Similar seasonal differences for stem xylem and soil water were observed for the δ2H values (Fig. 5). The data may, therefore, be used to investigate seasonal differences in root water uptake, infiltration of precipitation and snowmelt into the soil, evaporative enrichment of topsoil water, or to test models that simulate these processes.
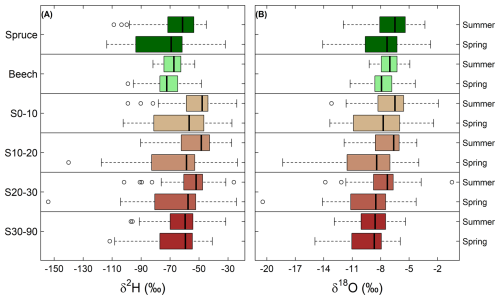
Figure 5Boxplots for the δ2H (A) and δ18O (B) values for stem xylem water of both tree species (beech and spruce) and soil water at 0–10 cm (S0–10), 10–20 cm (S10–20), 20–30 cm (S20–30) and 30–90 cm (S30–90) depth for the spring and summer campaigns. The vertical line within the box indicates the median (50th percentile). The box represents the interquartile range (IQR), spanning from the 25th percentile to the 75th percentile. The whiskers extend to the furthest data points within 1.5 times the IQR from the quartiles. Symbols outside the whiskers represent outliers.
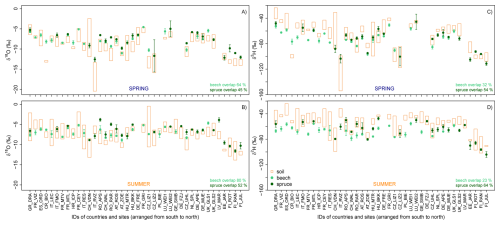
Figure 6The range in the isotopic composition of soil and stem xylem water for the spring (A, C) and summer (B, D) campaign for oxygen (δ18O) (left) and hydrogen (δ2H) (right). Orange bars indicate the minimum to maximum range for the soil water samples. Mean values and standard errors are shown for the isotopic composition of stem xylem water.
Further, we found that the isotopic composition of the stem xylem water plotted within the range of soil water at the site (“overlap”), though not consistently across all sites (Fig. 6). The mean δ18O values for the xylem water was within the variation of the soil water δ18O values for more beech sites (68 % in spring, 84 % in summer) than spruce sites (41 % in spring, 48 % in summer). The number of sites for which the δ18O values of the stem xylem water was within the range of soil water samples was larger for the summer than for the spring sampling campaign. In contrast, the mean δ2H values for the xylem water were within the range of the soil water samples for more spruce sites (58 % in spring, 68 % in summer) than beech sites (28 % in spring, 23 % summer). A lack of overlap may indicate that the trees used water from other sources, such as recent precipitation stored in organic surface layers, deeper, unsampled soil layers, or groundwater. Another explanation might be related to the spatial variation in the isotopic composition of the soil water, and cryogenic water extraction artefacts (see section on “Cryogenic water extraction biases”, Klein et al., 2014; Brinkmann et al., 2018; Knighton et al., 2020; Terzer-Wassmuth et al., 2023; Nelson et al., 2021; Allen et al., 2019; Floriancic et al., 2024a; Phillips and Gregg, 2003; Stock et al., 2018; Kirchner, 2023).
Our data also shows a clear isotopic difference in stem xylem water between the two tree species (Fig. 6). The mean species difference (spruce-beech) in δ2H and δ18O values across all sites was 5.5 ‰ and 0.8 ‰ in spring and 9.5 ‰ and 1.1 ‰ in summer, respectively. Thus, the stem xylem water in spruce tended to be isotopically enriched compared to beech xylem water, which is consistent with the generally shallower root system of spruce compared to beech (Goldsmith et al., 2019). The observed isotopic variability in stem xylem water among species and sites suggests that both species-specific differences in root water uptake depth and the environmental drivers of root water uptake across Europe can be inferred from these data.
These initial analyses suggest that the soil and stem xylem data can be used to test models that simulate plant-soil-water dynamics (Klein et al., 2014; Brinkmann et al., 2018; Knighton et al., 2020) and to test how this depends on site-, soil-, and tree-specific information (Table 3). When the data are combined with isotope data of precipitation, such as those from the GNIP network (e.g., Terzer-Wassmuth et al., 2023), or models, such as Piso.AI (Nelson et al., 2021), the data can also be used to study the seasonal origins of tree water uptake and its spatial and temporal variation (Allen et al., 2019; Floriancic et al., 2024a). For sites without overlap between the soil and xylem δ2H and δ18O values, the application of mixing models, such as IsoSource (Phillips and Gregg, 2003) or MixSIAR (Stock et al., 2018), might be limited. However, alternative mixing models with incomplete end-members could be tested (Kirchner, 2023)
4.2 Cryogenic water extraction biases
The dual isotope plots show that the isotope ratios of the soil were closer to the GMWL than those of stem xylem water for both species (Fig. 7). However, particularly in summer, the isotope ratios of the shallower soils at some locations also deviated from the GMWL. This may indicate that the water in the shallow soil was affected by evaporation and that the trees used this enriched water. While evaporation might be responsible for some of the offset between the soil and stem xylem samples, there was no evaporative enrichment for most soil samples. Nevertheless, it should be considered that soil organic matter can cause a bias in the isotopic composition of the extracted water (Ceperley et al., 2024; Orlowski et al., 2016), and that the presence of volatile organic compounds may interfere isotopic analysis with laser spectrometers (Martín-Gómez et al., 2015). The latter, however, should be reduced by the use of the micro-combustion module in our study. Given the relatively small differences between the laser and IRMS measurements (Fig. 3), the overall large deviation in δ2H from the GMWL for the stem xylem samples is more likely caused by methodological issues related to the cryogenic vacuum distillation method (Chen et al., 2020; Diao et al., 2022; Barbeta et al., 2022). According to these studies, biases might be related to stem water content, heterogeneity in the isotopic composition of different water pools in the stem xylem, the exchange of H-atoms between organic material and water or water vapour, and isotope fractionation related to evaporation and sublimation during the extraction.
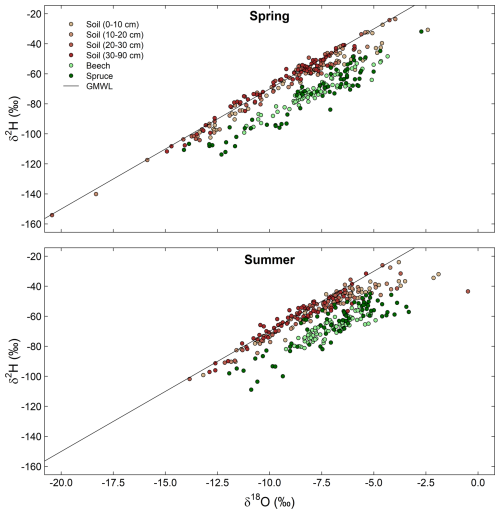
Figure 7Dual isotope plots of hydrogen and oxygen stable isotope ratios (δ2H, δ18O) for all soil and stem xylem water samples for the spring (top panel) and the summer (bottom panel) campaigns. Isotope values for soil samples are color coded according to soil depth. The line represents that Global Meteoric Water Line (GMWL): .
To assess potential systematic and technical influences on our data set, we performed several quality checks for cryogenic extraction and sample handling (Fig. 8). There was a significant difference in the total extraction efficiency for the samples handled by the three main lab technicians (one-way ANOVA, P<0.001; Fig. 8A), and this effect remained when accounting for site-level variation in a mixed-effects model. However, since each technician worked on samples for only one sampling season, the observed differences partially reflect seasonal effects, rather than lab technicians' performance alone. The total extraction efficiency did not depend on the cryogenic vacuum distillation slot (one-way ANOVA, P>0.05, Fig. 8B) and had a weak effect on the δ2H and δ18O values (Fig. 8C). Although samples with high versus low total extraction efficiency differed by ∼5 ‰ in δ2H and ∼0.5 ‰ in δ18O, linear regression showed that extraction efficiency explained less than 2 % of the variation in either isotope (R2<0.02, P>0.1).
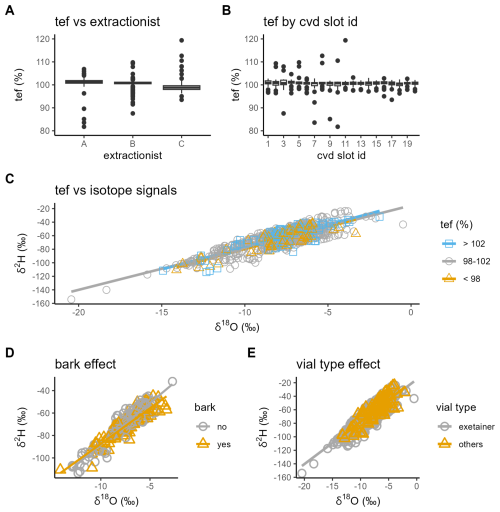
Figure 8Quality checks for cryogenic extraction and sampling handling. (A) Boxplot of the total extraction efficiency (tef, %) by lab technician (Person A, B, or C) and (B) by cryogenic vacuum distillation slot ID. Dual isotope plots color-coded by (C) tef category for all stem xylem and soil samples, (D) bark presence (“yes”) or absence (“no”) for stem xylem samples, and (E) vial type (“exetainer” vs. “others”) for all stem xylem and soil samples.
To further assess possible sample handling effects, we used linear mixed-effects models, including sampling campaign as a fixed effect and site ID as a random effect, to test the effect of bark presence and vial type (Fig. 8D and E). Interactions with sampling campaign were included due to uneven site numbers between spring and summer for bark (N=15 and 6) and vial type (N=23 and 4), respectively. While sampling campaign was a strong predictor (P<0.001), we observed no effect of bark presence on either isotope (P>0.05), nor any interaction with sampling campaign (P>0.05), suggesting that bark water was either isotopically similar to xylem water or present in insufficient quantity to alter the overall signal. In contrast, vial type significantly interacted with sampling campaign (P<0.001), with no effect in spring but a more depleted signal for the vial type “others” compared to “exetainer” for the summer sampling campaign. This pattern provides no indication of evaporative isotopic enrichment resulting from sample handling during the warmer summer conditions. Given that the “others” vial type comprises only ∼15 % of samples, spread across no more than 8 of 40 sites in both campaigns, we consider this effect unlikely to confound the overall dataset, though it may warrant consideration in future analyses. Collectively, these results support the overall reliability of the dataset and its suitability for analyses of cryogenic water extraction biases and methodological evaluation (Zhao et al., 2024; Sobota et al., 2024).
All data is freely available under the agreement “Creative Commons Zero – No Rights Reserved (CC0 1.0)” in the data repository EnviDat (Lehmann et al., 2024). https://doi.org/10.16904/envidat.542.
We present a large pan-European dataset of soil and stem xylem water isotopes for two common tree species collected during spring and summer 2023. Establishing this data set with a geographic cover across Europe was feasible because the participants took advantage of an EU Cost Action with members in most European countries. We believe that limiting the number of samples to 6 to 8 per site, along with regular progress updates through meetings or via email, contributed considerably to the success of the data collection. Centralizing the laboratory and analytical work avoided potential inter-laboratory biases, while the availability of an import license reduced shipping times and lowered the risk of sample loss. Since our observations are standardized according to recently published sampling and extraction procedures (Ceperley et al., 2024; Scandellari et al., 2024), this data can serve as a baseline for future ecohydrological studies. This dataset is freely available and represents a valuable resource for different research topics. These may include the identification of the factors that affect tree water uptake depth and the seasonal origin of the water used by trees, calibration and constraining isotope-aided ecohydrological models, isoscape models, or studying how biases caused by cryogenic water extraction vary by species, soil type, or climate.
Statistics
For all statistical analyses we used R version 4.3.1 (R Core Team, 2023). For the multiple linear regression analyses, we applied a cube root transformation to the data to address non-normality. We then used the R package “relaimpo” (Grömping, 2006) to assess the relative importance of the geographic characteristics in the model. Data presented for soil at a depth of 30–90 cm represents all available data points for soil depths greater than 30 cm, without any additional modifications of the data.
The supplement related to this article is available online at https://doi.org/10.5194/essd-17-6129-2025-supplement.
The WATSON sampling campaign core organization and writing team consisted of Marco M. Lehmann (MML), Josie Geris (JG), Ilja van Meerveld (IvM), Daniele Penna (DP), Youri Rothfuss (YR) and Katrin Meusburger (KM). The photographs were analysed by Matteo Verdone (MV). Conceptualization: MML, JG, IvM, DP, YR, KM; Data curation: MML, MV; Formal Analysis: MML, JG, IvM, DP, YR, MV, KM; Funding acquisition: MML, JG, IvM, DP, YR, KM; Investigation: MML, JG, IvM, DP, YR, KM; Methodology: MML, JG, IvM, DP, YR, KM; Project administration: MML, JG, IvM, DP, YR, KM; Resources: MML, KM; Validation: MML, JG, IvM, DP, YR, KM; Visualization: MML, JG, IvM, DP, YR, KM; Writing – original draft: MML, JG, IvM, DP, YR, KM; Writing – review and editing: all authors.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
The pan-European sampling campaign and the data collection initiative was developed during a workshop of the COST Action: “Water isotopes in the critical zone: from groundwater recharge to plant transpiration – WATSON” (https://watson-cost.eu/, last access: 6 November 2025) held in March 2023 in Dubrovnik, Croatia. We thank Timon Dufner, Christian Gruntz, Sophia Ezhold, Noemi Kammerlander, Jan Ziegler, Jonathan Frei, Roger Köchli, David Schweizer, Manuela Oettli for the laboratory assistance, as well as Enara Aldai, Wisam Almohamed, Hatice Türk, Patricia Vieira Pompeu, Fernanda Gianasi, Konstantinos Voudouris, Ionel Popa, Martine Helfer, Anna Meier, Ladina Gaudy, Dominik Gerber, Simon Bürki, Dominik Dubach, Paavo Ojanen, Ellinoora Ekman, Christiaan van der Tol, and Joni Koivula for their help with site selection, and/or sample- or metadata collection and laboratory assistance.
This study was financially supported by the COST Action: “WATSON” CA19120 (https://www.cost.eu/actions/CA19120, last access: 6 November 2025). The extraction of the water and isotope analyses were financially supported by the Swiss National Science Foundation (“TreeWater”, grant no. 205492; “InsightForest”, grant no. 213367) and by WSL (“Innovative project Oxygen17”). Alicia Correa was supported by the German Academic Exchange Service (DAAD) from funds of Federal Ministry for Economic Cooperation (BMZ), SDGnexus Network (grant no. 57526248). Aurel Persoiu and Andrei Popa were supported by UEFISCDI Romania (grant nos. PN-III-P2-2.1-PED-2019-4102 and PN-III-P4-ID-PCE-2020-2723). Maren Dubbert received funding by the Deutsche Forschungsgemeinschaft (grant no. 501530203) and by the Leibniz collaborative excellent grant (grant no. K444/2022), supporting Alberto Iraheta, Charlotte Koeber, and Clara Rohde. Katja Rinne was funded by the Academy of Finland (grant no. 343059). Research part at University of Oulu was supported by Research Council of Finland (grant nos. 347348 and 356043), and Marie Skłodowska-Curie Postdoctoral Fellowship (grant no. 101111527). Lukas Vlcek acknowledges funding by Czech Science Foundation (grant no. 22-12837S). Jiri Kocum was supported by the Czech Academy of Sciences (RVO: grant no. 67985874) and Faculty of Science, Charles University in Prague (SVV grant no. 244-260694).
This paper was edited by Birgit Heim and reviewed by three anonymous referees.
Allen, S. T., Kirchner, J. W., and Goldsmith, G. R.: Predicting spatial patterns in precipitation isotope (δ2H and δ18O) seasonality using sinusoidal isoscapes, Geophysical Research Letters, 45, 4859–4868, https://doi.org/10.1029/2018GL077458, 2018.
Allen, S. T., Kirchner, J. W., Braun, S., Siegwolf, R. T. W., and Goldsmith, G. R.: Seasonal origins of soil water used by trees, Hydrol. Earth Syst. Sci., 23, 1199–1210, https://doi.org/10.5194/hess-23-1199-2019, 2019.
Bachofen, C., Tumber-Dávila, S. J., Mackay, D. S., McDowell, N. G., Carminati, A., Klein, T., Stocker, B. D., Mencuccini, M., and Grossiord, C.: Tree water uptake patterns across the globe, New Phytol., 242, 1891–1910, https://doi.org/10.1111/nph.19762, 2024.
Barbeta, A., Burlett, R., Martín-Gómez, P., Fréjaville, B., Devert, N., Wingate, L., Domec, J. C., and Ogée, J.: Evidence for distinct isotopic compositions of sap and tissue water in tree stems: consequences for plant water source identification, New Phytol., 233, 1121–1132, https://doi.org/10.1111/nph.17857, 2022.
Beck, H. E., Mcvicar, T. R., Vergopolan, N., Berg, A., Lutsko, N. J., Dufour, A., Zeng, Z. Z., Jiang, X., van Dijk, A. I. J. M., and Miralles, D. G.: High-resolution (1 km) Köppen-Geiger maps for 1901–2099 based on constrained CMIP6 projections, Sci. Data, 10, https://doi.org/10.1038/s41597-023-02549-6, 2023.
Beyer, M. and Penna, D.: On the spatio-temporal under-representation of isotopic data in ecohydrological studies, Front. Water, 3, https://doi.org/10.3389/frwa.2021.643013, 2021.
Bowen, G. J.: Isoscapes: Spatial Pattern in Isotopic Biogeochemistry, Annual Review of Earth and Planetary Sciences, 38, 161–187, https://doi.org/10.1146/annurev-earth-040809-152429, 2010.
Bowling, D. R., Schulze, E. S., and Hall, S. J.: Revisiting streamside trees that do not use stream water: can the two water worlds hypothesis and snowpack isotopic effects explain a missing water source?, Ecohydrology, 10, e1771, https://doi.org/10.1002/eco.1771, 2017.
Brinkmann, N., Seeger, S., Weiler, M., Buchmann, N., Eugster, W., and Kahmen, A.: Employing stable isotopes to determine the residence times of soil water and the temporal origin of water taken up by Fagus sylvatica and Picea abies in a temperate forest, New Phytol., 219, 1300–1313, https://doi.org/10.1111/nph.15255, 2018.
Büntgen, U., Urban, O., Krusic, P. J., Rybníček, M., Kolář, T., Kyncl, T., Ač, A., Koňasová, E., Čáslavský, J., Esper, J., Wagner, S., Saurer, M., Tegel, W., Dobrovolný, P., Cherubini, P., Reinig, F., and Trnka, M.: Recent European drought extremes beyond Common Era background variability, Nat. Geosci., 14, 190–196, https://doi.org/10.1038/s41561-021-00698-0, 2021.
Bussotti, F., Potocic, N., Timmermann, V., Lehmann, M. M., and Pollastrini, M.: Tree crown defoliation in forest monitoring: concepts, findings, and new perspectives for a physiological approach in the face of climate change, Forestry, 97, 194–212, https://doi.org/10.1093/forestry/cpad066, 2024.
Cai, M. Y., Wang, L. X., Parkes, S. D., Strauss, J., McCabe, M. F., Evans, J. P., and Griffiths, A. D.: Stable water isotope and surface heat flux simulation using ISOLSM: Evaluation against in-situ measurements, J. Hydrol., 523, 67–78, https://doi.org/10.1016/j.jhydrol.2015.01.019, 2015.
Caudullo, G., Welk, E., and San-Miguel-Ayanz, J.: Chorological maps for the main European woody species, Data in Brief, 12, 662–666, https://doi.org/10.1016/j.dib.2017.05.007, 2017.
Ceperley, N., Gimeno, T. E., Jacobs, S. R., Beyer, M., Dubbert, M., Fischer, B., Geris, J., Holko, L., Kuebert, A., Le Gall, S., Lehmann, M. M., Llorens, P., Millar, C., Penna, D., Prieto, I., Radolinski, J., Scandellari, F., Stockinger, M., Stumpp, C., Tetzlaff, D., van Meerveld, I., Werner, C., Yildiz, O., Zuecco, G., Barbeta, A., Orlowski, N., and Rothfuss, Y.: Toward a common methodological framework for the sampling, extraction, and isotopic analysis of water in the Critical Zone to study vegetation water use, Wires Water, 11, https://doi.org/10.1002/wat2.1727, 2024.
Chen, Y. L., Helliker, B. R., Tang, X. H., Li, F., Zhou, Y. P., and Song, X.: Stem water cryogenic extraction biases estimation in deuterium isotope composition of plant source water, P. Natl. Acad. Sci. USA, 117, 33345–33350, https://doi.org/10.1073/pnas.2014422117, 2020.
Diao, H., Schuler, P., Goldsmith, G. R., Siegwolf, R. T. W., Saurer, M., and Lehmann, M. M.: Technical note: On uncertainties in plant water isotopic composition following extraction by cryogenic vacuum distillation, Hydrol. Earth Syst. Sci., 26, 5835–5847, https://doi.org/10.5194/hess-26-5835-2022, 2022.
Dubbert, M. and Werner, C.: Water fluxes mediated by vegetation: emerging isotopic insights at the soil and atmosphere interfaces, New Phytol., 221, 1754–1763, https://doi.org/10.1111/nph.15547, 2019.
Ellsworth, P. Z. and Sternberg, L. S. L.: Seasonal water use by deciduous and evergreen woody species in a scrub community is based on water availability and root distribution, Ecohydrology, 8, 538–551, https://doi.org/10.1002/eco.1523, 2015.
Engel, M., Frentress, J., Penna, D., Andreoli, A., van Meerveld, I., Zerbe, S., Tagliavini, M., and Comiti, F.: How do geomorphic characteristics affect the source of tree water uptake in restored river floodplains?, Ecohydrology, 15, e2443, https://doi.org/10.1002/eco.2443, 2022.
Erdélyi, D., Kern, Z., Nyitrai, T., and Hatvani, I. G.: Predicting the spatial distribution of stable isotopes in precipitation using amachine learning approach: a comparative assessment of random forest variants, Gem Int. J. Geomathema., 14, https://doi.org/10.1007/s13137-023-00224-x, 2023.
Fabiani, G., Penna, D., Barbeta, A., and Klaus, J.: Sapwood and heartwood are not isolated compartments: Consequences for isotope ecohydrology, Ecohydrology, 15, e2478, https://doi.org/10.1002/eco.2478, 2022.
Floriancic, M. G., Allen, S. T., and Kirchner, J. W.: Isotopic evidence for seasonal water sources in tree xylem and forest soils, Ecohydrology, https://doi.org/10.1002/eco.2641, 2024a.
Floriancic, M. G., Stockinger, M. P., Kirchner, J. W., and Stumpp, C.: Monthly new water fractions and their relationships with climate and catchment properties across Alpine rivers, Hydrol. Earth Syst. Sci., 28, 3675–3694, https://doi.org/10.5194/hess-28-3675-2024, 2024b.
Gessler, A., Bachli, L., Freund, E. R., Treydte, K., Schaub, M., Haeni, M., Weiler, M., Seeger, S., Marshall, J., Hug, C., Zweifel, R., Hagedorn, F., Rigling, A., Saurer, M., and Meusburger, K.: Drought reduces water uptake in beech from the drying topsoil, but no compensatory uptake occurs from deeper soil layers, New Phytol., 233, 194–206, https://doi.org/10.1111/nph.17767, 2022.
Goldsmith, G. R., Allen, S. T., Braun, S., Engbersen, N., Gonzalez-Quijano, C. R., Kirchner, J. W., and Siegwolf, R. T. W.: Spatial variation in throughfall, soil, and plant water isotopes in a temperate forest, Ecohydrology, 12, https://doi.org/10.1002/Eco.2059, 2019.
Grömping, U.: Relative Importance for Linear Regression in R: The Package relaimpo, Journal of Statistical Software, 17, 1–27, 2006.
Haese, B., Werner, M., and Lohmann, G.: Stable water isotopes in the coupled atmosphere–land surface model ECHAM5-JSBACH, Geosci. Model Dev., 6, 1463–1480, https://doi.org/10.5194/gmd-6-1463-2013, 2013.
Halder, J., Terzer, S., Wassenaar, L. I., Araguás-Araguás, L. J., and Aggarwal, P. K.: The Global Network of Isotopes in Rivers (GNIR): integration of water isotopes in watershed observation and riverine research, Hydrol. Earth Syst. Sci., 19, 3419–3431, https://doi.org/10.5194/hess-19-3419-2015, 2015.
Kinzinger, L., Mach, J., Haberstroh, S., Schindler, Z., Frey, J., Dubbert, M., Seeger, S., Seifert, T., Weiler, M., Orlowski, N., Werner, C., and Meinzer, F.: Interaction between beech and spruce trees in temperate forests affects water use, root water uptake pattern and canopy structure, Tree Physiology, 44, https://doi.org/10.1093/treephys/tpad144, 2024.
Kirchner, J. W.: Mixing models with multiple, overlapping, or incomplete end-members, quantified using time series of a single tracer, Geophysical Research Letters, 50, https://doi.org/10.1029/2023GL104147, 2023.
Klein, T., Rotenberg, E., Cohen-Hilaleh, E., Raz-Yaseef, N., Tatarinov, F., Preisler, Y., Ogée, J., Cohen, S., and Yakir, D.: Quantifying transpirable soil water and its relations to tree water use dynamics in a water-limited pine forest, Ecohydrology, 7, 409–419, https://doi.org/10.1002/eco.1360, 2014.
Knighton, J., Kuppel, S., Smith, A., Soulsby, C., Sprenger, M., and Tetzlaff, D.: Using isotopes to incorporate tree water storage and mixing dynamics into a distributed ecohydrologic modelling framework, Ecohydrology, 13, https://doi.org/10.1002/eco.2201, 2020.
Koeniger, P., Stumpp, C., and Schmidt, A.: Stable isotope patterns of German rivers with aspects on scales, continuity and network status, Isotopes in Environmental and Health Studies, 58, 363–379, https://doi.org/10.1080/10256016.2022.2127702, 2022.
Lehmann, M. M., Geris, J., van Meerveld, I., Penna, D., Rothfuss, Y., Verdone, M., Ala-Aho, P., Arvai, M., Babre, A., Balandier, P., Bernhard, F., Butorac, L., Carrière, S. D., Ceperley, N. C., Chen, Z., Correa, A., Diao, H., Dubbert, D., Dubbert, M., Ercoli, F., Floriancic, M. G., Gimeno, T. E., Gounelle, D., Hagedorn, F., Hissler, C., Huneau, F., Alberto, I., Jakovljević, T., Kazakis, N., Kern, Z., Knaebel, K., Kobler, J., Kocum, J., Koeber, C., Koren, G., Kübert, A., Kupka, D., le Gall, S., Lehtonen, A., Leydier, T., Malagoli, P., Manca di Villahermosa, F. S., Marchina, C., Martínez-Carreras, N., Martin-StPaul, N., Marttila, H., Meyer Oliveira, A., Monvoisin, G., Orlowski, N., Palmik-Das, K., Persoiu, A., Popa, A., Prikaziuk, E., Quantin, C., Rinne-Garmston, K. T., Rohde, C., Sanda, M., Saurer, M., Schulz, D., Stockinger, M. P., Stumpp, C., Vénisse, J.-S., Vlcek, L., Voudouris, S., Weeser, B., Wilkinson, M., Zuecco, G., and Meusburger, K.: Soil and stem xylem water isotope data from two pan-European sampling campaigns [data set], https://doi.org/10.16904/envidat.542, 2024.
Lindner, M., Maroschek, M., Netherer, S., Kremer, A., Barbati, A., Garcia-Gonzalo, J., Seidl, R., Delzon, S., Corona, P., Kolström, M., Lexer, M. J., and Marchetti, M.: Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems, Forest Ecology and Management, 259, 698–709, https://doi.org/10.1016/j.foreco.2009.09.023, 2010.
Martín-Gómez, P., Barbeta, A., Voltas, J., Peñuelas, J., Dennis, K., Palacio, S., Dawson, T. E., and Ferrio, J. P.: Isotope-ratio infrared spectroscopy: a reliable tool for the investigation of plant-water sources?, New Phytol., 207, 914–927, https://doi.org/10.1111/nph.13376, 2015.
Nelson, D. B., Basler, D., and Kahmen, A.: Precipitation isotope time series predictions from machine learning applied in Europe, P. Natl. Acad. Sci. USA, 118, e2024107118, https://doi.org/10.1073/pnas.2024107118, 2021.
Orlowski, N., Breuer, L., and McDonnell, J. J.: Critical issues with cryogenic extraction of soil water for stable isotope analysis, Ecohydrology, 9, 1–5, https://doi.org/10.1002/eco.1722, 2016.
Orlowski, N., Breuer, L., Angeli, N., Boeckx, P., Brumbt, C., Cook, C. S., Dubbert, M., Dyckmans, J., Gallagher, B., Gralher, B., Herbstritt, B., Hervé-Fernández, P., Hissler, C., Koeniger, P., Legout, A., Macdonald, C. J., Oyarzún, C., Redelstein, R., Seidler, C., Siegwolf, R., Stumpp, C., Thomsen, S., Weiler, M., Werner, C., and McDonnell, J. J.: Inter-laboratory comparison of cryogenic water extraction systems for stable isotope analysis of soil water, Hydrol. Earth Syst. Sci., 22, 3619–3637, https://doi.org/10.5194/hess-22-3619-2018, 2018.
Orlowski, N., Rinderer, M., Dubbert, M., Ceperley, N., Hrachowitz, M., Gessler, A., Rothfuss, Y., Sprenger, M., Heidbüchel, I., Kübert, A., Beyer, M., Zuecco, G., and McCarter, C.: Challenges in studying water fluxes within the soil-plant-atmosphere continuum: A tracer-based perspective on pathways to progress, Sci. Total Environ., 881, https://doi.org/10.1016/j.scitotenv.2023.163510, 2023.
Penna, D., Stenni, B., Šanda, M., Wrede, S., Bogaard, T. A., Michelini, M., Fischer, B. M. C., Gobbi, A., Mantese, N., Zuecco, G., Borga, M., Bonazza, M., Sobotková, M., Čejková, B., and Wassenaar, L. I.: Technical Note: Evaluation of between-sample memory effects in the analysis of δ2H and δ18O of water samples measured by laser spectroscopes, Hydrol. Earth Syst. Sci., 16, 3925–3933, https://doi.org/10.5194/hess-16-3925-2012, 2012.
Penna, D., Hopp, L., Scandellari, F., Allen, S. T., Benettin, P., Beyer, M., Geris, J., Klaus, J., Marshall, J. D., Schwendenmann, L., Volkmann, T. H. M., von Freyberg, J., Amin, A., Ceperley, N., Engel, M., Frentress, J., Giambastiani, Y., McDonnell, J. J., Zuecco, G., Llorens, P., Siegwolf, R. T. W., Dawson, T. E., and Kirchner, J. W.: Ideas and perspectives: Tracing terrestrial ecosystem water fluxes using hydrogen and oxygen stable isotopes – challenges and opportunities from an interdisciplinary perspective, Biogeosciences, 15, 6399–6415, https://doi.org/10.5194/bg-15-6399-2018, 2018.
Penna, D., Geris, J., Hopp, L., and Scandellari, F.: Water sources for root water uptake: Using stable isotopes of hydrogen and oxygen as a research tool in agricultural and agroforestry systems, Agr. Ecosyst. Environ., 291, https://doi.org/10.1016/j.agee.2019.106790, 2020.
Peters, R. L., Speich, M., Pappas, C., Kahmen, A., von Arx, G., Pannatier, E. G., Steppe, K., Treydte, K., Stritih, A., and Fonti, P.: Contrasting stomatal sensitivity to temperature and soil drought in mature alpine conifers, Plant, Cell & Environment, 42, 1674–1689, https://doi.org/10.1111/pce.13500, 2019.
Phillips, D. L. and Gregg, J. W.: Source partitioning using stable isotopes: coping with too many sources, Oecologia, 136, 261–269, https://doi.org/10.1007/s00442-003-1218-3, 2003.
Poca, M., Coomans, O., Urcelay, C., Zeballos, S. R., Bodé, S., and Boeckx, P.: Isotope fractionation during root water uptake by is enhanced by arbuscular mycorrhizas, Plant Soil, 441, 485–497, https://doi.org/10.1007/s11104-019-04139-1, 2019.
R Core Team: R: A language and environment for statistical computing, R foundation for statistical computing, Vienna, Austria, https://www.r-project.org/, last access: 6 November 2025, 2023.
Reckerth, A., Stichler, W., Schmidt, A., and Stumpp, C.: Long-term data set analysis of stable isotopic composition in German rivers, J. Hydrol., 552, 718–731, https://doi.org/10.1016/j.jhydrol.2017.07.022, 2017.
Rothfuss, Y. and Javaux, M.: Reviews and syntheses: Isotopic approaches to quantify root water uptake: a review and comparison of methods, Biogeosciences, 14, 2199–2224, https://doi.org/10.5194/bg-14-2199-2017, 2017.
Scandellari, F., Attou, T., Barbeta, A., Bernhard, F., D'Amato, C., Dimitrova-Petrova, K., Donaldson, A., Durodola, O., Ferraris, S., Floriancic, M. G., Fontenla-Razzetto, G., Gerchow, M., Han, Q., Khalil, I., Kirchner, J. W., Kühnhammer, K., Liu, Q., Llorens, P., Magh, R. K., Marshall, J., Meusburger, K., Oliveira, A. M., Muñoz-Villers, L., Pires, S. S., Todini-Zicavo, D., van Meerveld, I., Voigt, C., Wirsig, L., Beyer, M., Geris, J., Hopp, L., Penna, D., and Sprenger, M.: Using stable isotopes to inform water resource management in forested and agricultural ecosystems, J. Environ. Manage., 365, https://doi.org/10.1016/j.jenvman.2024.121381, 2024.
Sobota, M., Li, K. V., Hren, M., and Knighton, J.: Evidence for variations in cryogenic extraction deuterium biases of plant xylem water across foundational northeastern US trees, Hydrol. Process., 38, https://doi.org/10.1002/hyp.15079, 2024.
Spinoni, J., Naumann, G., Carrao, H., Barbosa, P., and Vogt, J.: World drought frequency, duration, and severity for 1951–2010, Int. J. Climatol., 34, 2792–2804, https://doi.org/10.1002/joc.3875, 2014.
Stock, B. C., Jackson, A. L., Ward, E. J., Parnell, A. C., Phillips, D. L., and Semmens, B. X.: Analyzing mixing systems using a new generation of Bayesian tracer mixing models, Peerj, 6, https://doi.org/10.7717/peerj.5096, 2018.
Terzer, S., Wassenaar, L. I., Araguás-Araguás, L. J., and Aggarwal, P. K.: Global isoscapes for δ18O and δ2H in precipitation: improved prediction using regionalized climatic regression models, Hydrol. Earth Syst. Sci., 17, 4713–4728, https://doi.org/10.5194/hess-17-4713-2013, 2013.
Terzer-Wassmuth, S., Araguás-Araguás, L. J., Wassenaar, L. I., and Stumpp, C.: Global and local meteoric water lines for and the spatiotemporal distribution of Δ′17O in Earth's precipitation, Sci. Rep.-UK, 13, https://doi.org/10.1038/s41598-023-45920-8, 2023.
Twining, J., Stone, D., Tadros, C., Henderson-Sellers, A., and Williams, A.: Moisture Isotopes in the Biosphere and Atmosphere (MIBA) in Australia: A priori estimates and preliminary observations of stable water isotopes in soil, plant and vapour for the Tumbarumba Field Campaign, Global Planet. Change, 51, 59–72, https://doi.org/10.1016/j.gloplacha.2005.12.005, 2006.
von Freyberg, J., Allen, S. T., Seeger, S., Weiler, M., and Kirchner, J. W.: Sensitivity of young water fractions to hydro-climatic forcing and landscape properties across 22 Swiss catchments, Hydrol. Earth Syst. Sci., 22, 3841–3861, https://doi.org/10.5194/hess-22-3841-2018, 2018.
Wong, T. E., Nusbaumer, J., and Noone, D. C.: Evaluation of modeled land-atmosphere exchanges with a comprehensive water isotope fractionation scheme in version 4 of the Community Land Model, J. Adv. Model. Earth Sy., 9, 978–1001, https://doi.org/10.1002/2016ms000842, 2017.
Zhao, L. J., Liu, X. H., Wang, N. L., Barbeta, A., Zhang, Y., Cernusak, L. A., and Wang, L. X.: The determining factors of hydrogen isotope offsets between plants and their source waters, New Phytol., 241, 2009–2024, https://doi.org/10.1111/nph.19492, 2024.