the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Artemisia pollen dataset for exploring the potential ecological indicators in deep time
Li-Li Lu
Bo-Han Jiao
Feng Qin
Gan Xie
Kai-Qing Lu
Jin-Feng Li
Bin Sun
Min Li
David K. Ferguson
Tian-Gang Gao
Yi-Feng Yao
Artemisia, along with Chenopodiaceae, is the dominant component growing in the desert and dry grassland of the Northern Hemisphere. Artemisia pollen with its high productivity, wide distribution, and easy identification is usually regarded as an eco-indicator for assessing aridity and distinguishing grassland from desert vegetation in terms of the pollen relative abundance ratio of ChenopodiaceaeArtemisia (CA). Nevertheless, divergent opinions on the degree of aridity evaluated by Artemisia pollen have been circulating in the palynological community for a long time. To solve the confusion, we first selected 36 species from nine clades and three outgroups of Artemisia based on the phylogenetic framework, which attempts to cover the maximum range of pollen morphological variation. Then, sampling, experiments, photography, and measurements were taken using standard methods. Here, we present pollen datasets containing 4018 original pollen photographs, 9360 pollen morphological trait measurements, information on 30 858 source plant occurrences, and corresponding environmental factors. Hierarchical cluster analysis on pollen morphological traits was carried out to subdivide Artemisia pollen into three types. When plotting the three pollen types of Artemisia onto the global terrestrial biomes, different pollen types of Artemisia were found to have different habitat ranges. These findings change the traditional concept of Artemisia being restricted to arid and semi-arid environments. The data framework that we designed is open and expandable for new pollen data of Artemisia worldwide. In the future, linking pollen morphology with habitat via these pollen datasets will create additional knowledge that will increase the resolution of the ecological environment in the geological past. The Artemisia pollen datasets are freely available at Zenodo (https://doi.org/10.5281/zenodo.6900308; Lu et al., 2022).
- Article
(23270 KB) - Full-text XML
- BibTeX
- EndNote
The concept of global change can be considered as any consistent trend in the environment – past, present, or projected – that affects a substantial part of the globe. Consequently, past climates shed light on our future (Tierney et al., 2020). When attempting to reconstruct past global change prior to meteorological records, we need some appropriate biological or abiotic proxies based on long-term, consistently collected data, e.g. leaf wax biomarkers (Bhattacharya et al., 2018), tree-ring data (Moberg et al., 2005), leaf form (Yang et al., 2015), pollen data (Mosbrugger et al., 2005; Guiot and Cramer, 2016; Marsicek et al., 2018), atmospheric carbon dioxide (Zachos et al., 2008; Beerling and Royer, 2011), and isotope records (Zachos et al., 2001; Sánchez-Murillo et al., 2019). Determining a suitable proxy to reconstruct palaeoclimate and palaeoenvironment is a great scientific challenge (Tierney et al., 2020; McClelland et al., 2021).
The pollen of Artemisia (A), together with that of Chenopodiaceae (C) in arid and semi-arid areas, in the form of the ratio of CA pollen abundance, was applied to distinguish grassland and desert vegetation types and assess the degree of drought in the geological past (El-Moslimany, 1990; Sun et al., 1994; Davies and Fall, 2001; Herzschuh et al., 2004; Xu et al., 2007; Zhao et al., 2009, 2012; Zhang et al., 2010; Li et al., 2017; Ma et al., 2017; Koutsodendris et al., 2019; Wang et al., 2020), because both Chenopodiaceae and Artemisia are dominant elements of desert vegetation (Wu, 1980; Vrba, 1980; Tarasov et al., 1998; Herzschuh et al., 2004; Li et al., 2010; Zhao et al., 2021), and the sum of their pollen relative abundances in the surface soil is usually more than 50 % in arid and semi-arid areas (Sun et al., 1994; Lu et al., 2020).
Among them, the pollen of Artemisia, with its high productivity, wide spatial and temporal distribution, easy identification, and morphological uniformity under the light microscope (LM), is an essential component and useful bio-indicator in pollen-based past vegetation reconstructions and environmental assessments. Some researchers regarded Artemisia as an aridity indicator (El-Moslimany, 1990; Yi et al., 2003a, b; Liu et al., 2006; Cai et al., 2019; Cui et al., 2019; Chen et al., 2020; Wu et al., 2020; Cao et al., 2021), while others suggested that the correlation between the relative abundance of Artemisia pollen and humidity was insignificant (Weng et al., 1993; Sun et al., 1996; Koutsodendris et al., 2019; Lu et al., 2020; Zhao et al., 2021). Consequently, there is an urgent need to evaluate whether different pollen types of Artemisia represent distinct habitats.
In the past, Artemisia pollen was regarded as very uniform under LM (Wodehouse, 1926; Sing and Joshi, 1969; Ling, 1982; Wang et al., 1995). For instance, following the description and statistics of pollen morphology of 27 species of Artemisia in Eurasia under LM, Sing and Joshi (1969) stated that the pollen grains of Artemisia are consistent and continuous in morphology. Later, some authors recognized a series of pollen types (Chen, 1987; Jiang et al., 2005; Ghahraman et al., 2007; Shan et al., 2007; Hayat et al., 2009, 2010; Hussain et al., 2019) based on a detailed survey of the pollen micromorphology of different taxa under the scanning electron microscope (SEM).
For example, Chen (1987) described the pollen morphology of 77 Artemisia species from China under LM and SEM and divided these pollen grains into six types by using pollen characters, such as the shape and size of the spinules and density of spinules and granules. Type I (sparse spinules with granules among them), type II (dense spinules, no or few granules), type III (sparse spinules, no granules), type IV (dense spinules, well-developed granules), type V (small and sparse spinules, smooth tectum), and type VI (dissimilar spinules with granules among them).
Shan et al. (2007) investigated the pollen morphology of 32 Artemisia species from the Loess Plateau of China under LM and SEM and divided these pollen grains into five types according to exine sculpture: type I (dense spinules with swollen bases, small granules), type II (dense spinules, swollen bases almost united), type III (dense spinules with swollen bases and smooth tectum), type IV (sparse small spinules and smooth tectum), and type V (sparse spinules, small granules).
Jiang et al. (2005) observed the pollen morphology of 57 representative plants in seven groups of Artemisia under LM and SEM. This pollen can be divided into two types based on exine sculpture: type I (spinules multi-ruminated with flared bases, connecting the mostly densely arranged spinules) and type II (densely or loosely arranged spinules without flared bases, interspace glandular or smooth) with subtypes II-1, II-2, II-3, and II-4 based on the distribution of the spinules.
Ghahraman et al. (2007) studied the pollen morphology of 26 species of the 33 Artemisia species in Iran under LM and SEM. Based on exine ornamentation observed under SEM, two types of pollen grains were recognized: type I, exine surface covered with dense acute spinules, and type II, exine surface with few spinules.
Hayat et al. (2009, 2010) carried out a palynological study of 22 Artemisia species from Pakistan under LM and SEM. Earlier work demonstrated the phylogenetic associations within Artemisia based on a phylogenetic analysis of nine characters (pollen type, pollen shape, spinule arrangement, exine sculpture, spinule base, the length of polar axis, the length of equatorial axis, exine thickness, and colpus width) of pollen grains of Artemisia. In the latter work, eight micromorphological characters were identified and pooled by cluster analysis, leading to the recognition of five groups.
Hussain et al. (2019) studied the pollen morphology of 15 Artemisia species in the Gilgit–Baltistan region of Pakistan utilizing SEM and divided these species into four groups based on cluster analysis of seven micromorphological characters (pollen type, pollen shape, spinule arrangement, exine sculpture, spinule base, polar length, and equatorial width).
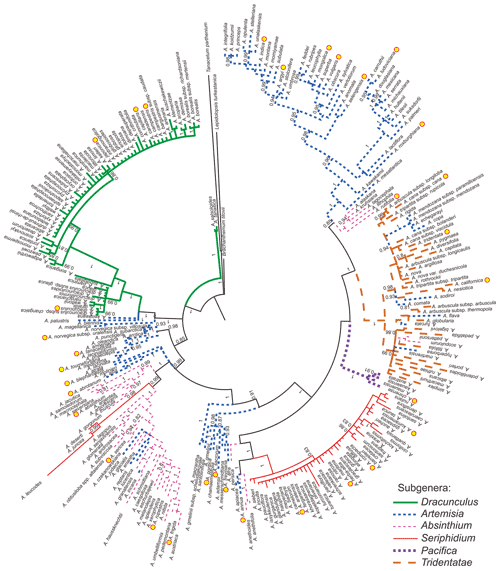
Figure 1Phylogenetic tree of Artemisia (modified from Malik et al., 2017). The styles of the strokes that were used to draw the branches indicate the traditional subgeneric classification of Artemisia and the yellow spots indicate sampled taxa.
Almost all of the above-mentioned Artemisia pollen classifications were designed to solve taxonomic or phylogenetic problems, and only a few were concerned with linking diverse habitats to the different pollen types in Artemisia.
Here, we attempt to (1) present abundant pollen photographs of 36 species from nine branches and three outgroups of the genus (ca. 400 species worldwide, see Ling, 1982; Bremer and Humphries, 1993), constrained by the phylogenetic framework of Artemisia (Sanz et al., 2008; Malik et al., 2017); (2) describe and measure the morphological traits of these pollen grains; (3) provide a new classification of pollen types and their distribution worldwide, with a key to pollen types in Artemisia; and (4) explore the diverse ecological niches of Artemisia represented by different pollen types in order to evaluate palaeovegetation and reconstruct palaeoenvironments.
2.1 Sampling strategy
The 36 pollen samples studied were selected from voucher sheets in the PE herbarium at the Institute of Botany, Chinese Academy of Sciences (Fig. 1 and Table B1), covering nine main clades, i.e. subg. Tridentata, subg. Artemisia (contains Sect. Artemisia, Sect. Abrotanum I, Sect. Abrotanum II, and Sect. Abrotanum III), subg. Pacifica, subg. Seriphidium, subg. Absinthium, and subg. Dracunculus, constrained by the phylogenetic framework of Artemisia (Malik et al., 2017) and three outgroups (Sanz et al., 2008), reflecting the maximum diversity or morphological variation under LM and SEM.
2.2 Data acquisition
Pollen samples were acetolysed by the standard method (Erdtman, 1960) and fixed in glycerine jelly. Standard procedures were followed for LM and SEM (Chen, 1987; Wang et al., 1995). The pollen grains were photographed under LM (Leica DM 4000) at a magnification of ×1000 and SEM (Hitachi S-4800) at an accelerating voltage of 30 kV. The pollen terminology followed the descriptions of Hesse et al. (2009) and Halbritter et al. (2018). The statistical pollen morphological traits under LM (Fig. 2a and b, P: polar length; E: equatorial width; ; T: exine thickness; L: pollen length; ) of each species were measured using 20 pollen grains. We chose five pollen grains under SEM for each exine ornamentation trait in each species (Fig. 2c–f, D: diameter of spinule base; H: spinule height; ; Gs: granule spacing; Ss: Spinule spacing; ; Ps: perforation spacing) and, on average, randomly selected four regions of each pollen grain for measuring, yielding a total of 20 measurements. The mean value (M) and standard deviation (SD) of the pollen grains of each species were measured and calculated in both polar and equatorial views (Appendix A and Table 1).
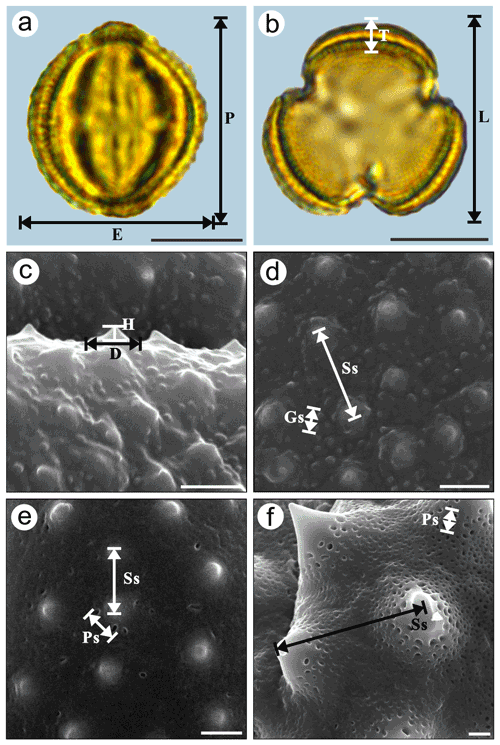
Figure 2Graphical illustration of measured pollen morphological traits in Artemisia (a, b: A. annua; c, d: A. vulgaris) and outgroups (e: Kaschagaria brachanthemoides; and f: Ajania pallasiana). Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
The scientific names of selected taxa were standardized according to Plants of the World Online (https://powo.science.kew.org/, last access: 21 November 2021). The specimen sampling coordinates of the corresponding taxa were obtained from the Global Biodiversity Information Facility (GBIF, https://www.gbif.org/, last access: 9 November 2021). Only preserved specimens were filtered for GBIF data given their well-documented geographical information and the availability of specimens as definitive vouchers. The distribution data on observations and cultivated collections provided by GBIF were excluded because they may contain incorrect identification or incorrect geo-referencing (Brummitt et al., 2021). Next, the distribution data were standardized cleaned using R package “CoordinateCleaner” (Zizka et al., 2019); no outliers were found.
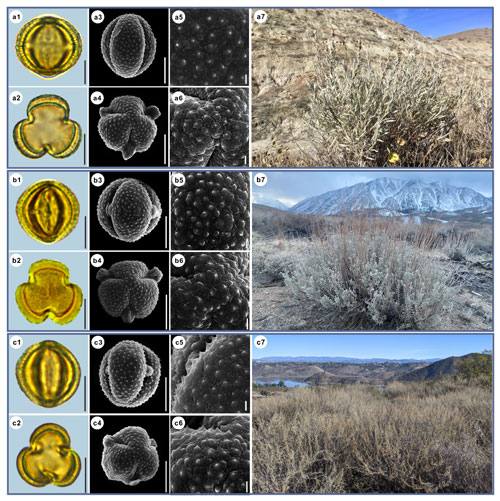
Figure 3Pollen grains and the habitats of their source plants. (a) Artemisia cana; (b) Artemisia tridentata; and (c) Artemisia californica. Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 cited from https://www.inaturalist.org/photos/54492753, last access: 19 August 2022, by © Jason Headley, b7 cited from https://www.inaturalist.org/photos/117436654, last access: 19 August 2022, by © Matt Berger, c7 cited from https://www.inaturalist.org/photos/108921528, last access: 19 August 2022, by © Don Rideout). Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
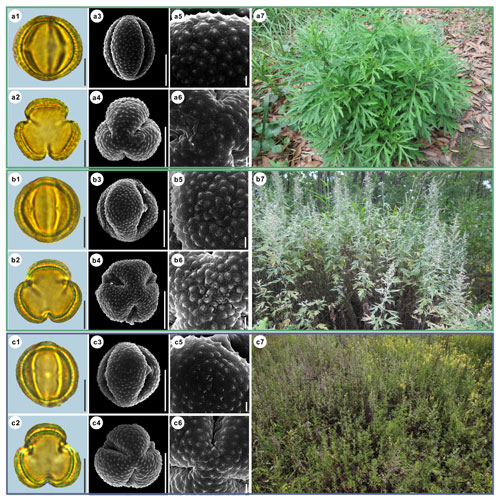
Figure 4Pollen grains and the habitats of their source plants. (a) Artemisia indica; (b) Artemisia argyi; and (c) Artemisia mongolica. Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 cited from https://www.inaturalist.org/photos/66336449, last access: 19 August 2022, by © yangting, b7 cited from https://www.inaturalist.org/photos/95820686, last access: 19 August 2022, by © Sergey Prokopenko, c7 cited from https://www.inaturalist.org/photos/163584035, last access: 19 August 2022, by © Nikolay V Dorofeev). Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
The corresponding environmental factors including altitude and 19 climate parameters of these coordinates were obtained from WorldClim (https://www.worldclim.org/, last access: 15 May 2017) with a spatial resolution of 30 s (∼1 km2) in 1970–2000 by Extract MultiValues To Points using ArcGIS 10.2 software in bilinear interpolation.
2.3 Data processing
OriginPro 2021 software was used for hierarchical cluster analysis on Artemisia and its outgroup pollen data. The Euclidean distance was calculated after the normalization of the original data, and the Ward method was used for clustering. Five groups were established, and the centre point of each group was calculated according to the sum of distances. Pollen morphological traits for the principal component analysis (PCA) of Artemisia and its outgroups were grouped according to the five groups of the cluster analysis. OriginPro 2021 software was used to draw group violin diagrams and boxplots respectively, to run an ANOVA to test for an overall difference between the pollen characters of three pollen types, and to test intraspecific variability in pollen exine ultrastructure characters among three representative species, followed by post hoc tests (Tukey). OriginPro 2021 software was also used to draw group violin diagrams and run a Kruskal–Wallis ANOVA to test for overall differences between the environmental factors of the three pollen types. The images of habitats reproduced in the text are from the websites listed in Table B1.
The global distribution data of the 36 representative species and three pollen types were plotted on the map of terrestrial ecological regions (Olson et al., 2001) using ArcGIS 10.2 software (Figs. 16 and 21).
3.1 Artemisia pollen grains and their source plant habitats
Here, we provide detailed data on pollen morphological traits, covering 36 species from nine main clades of Artemisia and three outgroups constrained by the phylogenetic framework (Fig. 1, Sanz et al., 2008; Malik et al., 2017) under LM and SEM, and the habitats of their source plants (Figs. 3–14).
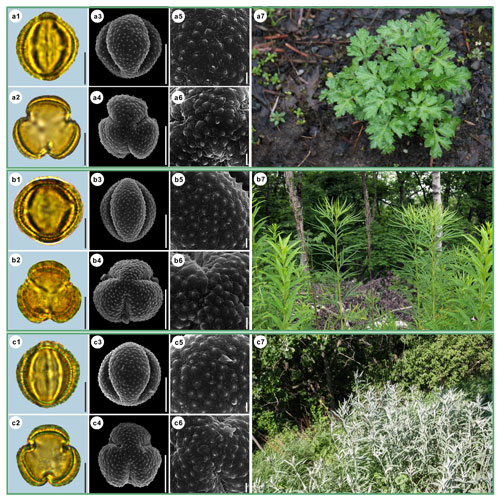
Figure 5Pollen grains and the habitats of their source plants. (a) Artemisia vulgaris; (b) Artemisia selengensis; and (c) Artemisia ludoviciana. Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 cited from https://www.inaturalist.org/photos/120600448, last access: 19 August 2022, by © Sara Rall, b7 cited from https://www.inaturalist.org/photos/46352423, last access: 19 August 2022, by © Gularjanz Grigoryi Mihajlovich, c7 cited from https://www.inaturalist.org/photos/77690333, last access: 19 August 2022, by © Ethan Rose). Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.

Figure 6Pollen grains and the habitats of their source plants. (a) Artemisia roxburghiana; (b) Artemisia rutifolia; and (c) Artemisia chinensis. Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 provided by © Bo-Han Jiao, b7 cited from https://www.inaturalist.org/photos/62207191, last access: 19 August 2022, by © Daba, c7 provided by © Jia-Hao Shen). Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
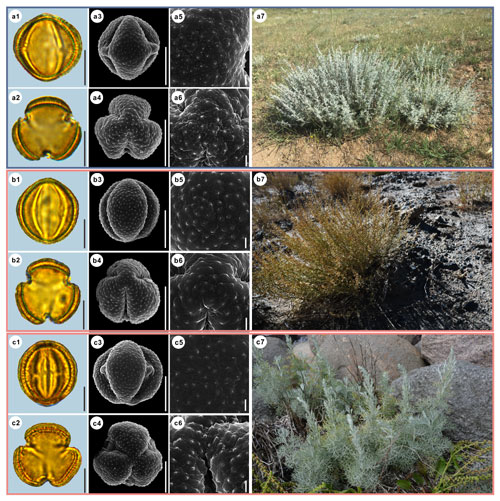
Figure 7Pollen grains and the habitats of their source plants. (a) Artemisia kurramensis; (b) Artemisia compactum; and (c) Artemisia maritima. Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 cited from https://www.inaturalist.org/photos/133758174, last access: 19 August 2022, by © Andrey Vlasenko, b7 provided by © Chen Chen, c7 cited from https://www.inaturalist.org/photos/86515371, last access: 19 August 2022, by © torkild). Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
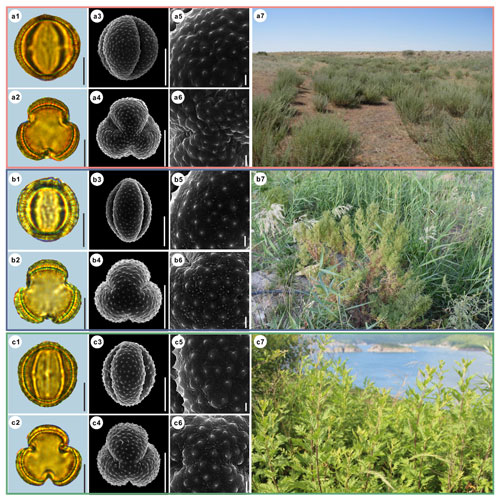
Figure 8Pollen grains and the habitats of their source plants.
(a) Artemisia aralensis; (b) Artemisia annua; and (c) Artemisia freyniana.
Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 cited from
https://www.plantarium.ru/lang/en/page/image/id/73063.html, last access: 19 August 2022, by
© ![]()
![]() , b7 provided by © Chen Chen, c7 cited
from https://www.inaturalist.org/photos/154390279, last access: 19 August 2022, by ©
, b7 provided by © Chen Chen, c7 cited
from https://www.inaturalist.org/photos/154390279, last access: 19 August 2022, by ©
![]()
![]() ).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.

Figure 9Pollen grains and the habitats of their source plants. (a) Artemisia stechmanniana; (b) Artemisia pontica; and (c) Artemisia frigida. Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 provided by © Bo-Han Jiao, b7 cited from https://www.inaturalist.org/photos/93438780, last access: 19 August 2022, by © Martin Pražák, c7 cited from https://www.inaturalist.org/photos/125022240, last access: 19 August 2022, by © Suzanne Dingwell). Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
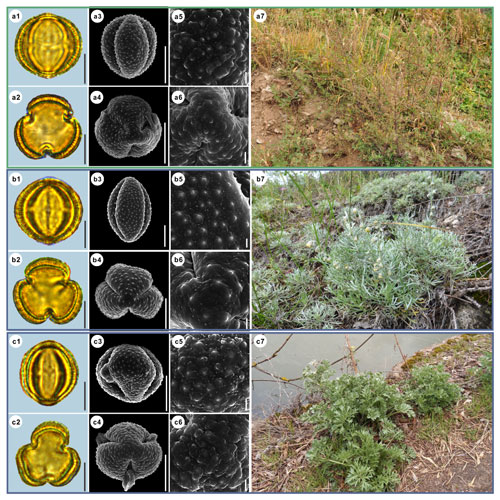
Figure 10Pollen grains and the habitats of their source plants.
(a) Artemisia rupestris; (b) Artemisia sericea; and (c) Artemisia absinthium.
Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 provided by
© Bo-Han Jiao, b7 cited from https://www.inaturalist.org/photos/48033353, last access: 19 August 2022, by ©
svetlana_katana, c7 cited from https://www.inaturalist.org/photos/123569286, last access: 19 August 2022, by © ![]() ).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
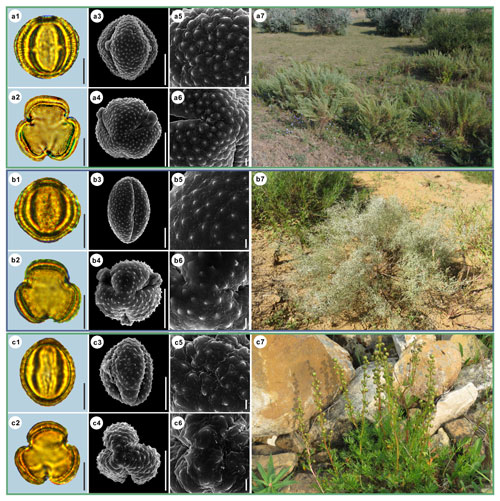
Figure 11Pollen grains and the habitats of their source plants.
(a) Artemisia abrotanum; (b) Artemisia blepharolepis; and (c) Artemisia norvegica.
Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 cited from
https://www.inaturalist.org/photos/116106722, last access: 19 August 2022, by ©
![]() , b7 provided by © Ji-Ye Zheng, c7 cited from
https://www.inaturalist.org/photos/161393521, last access: 19 August 2022, by © Erin
Springinotic).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
, b7 provided by © Ji-Ye Zheng, c7 cited from
https://www.inaturalist.org/photos/161393521, last access: 19 August 2022, by © Erin
Springinotic).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
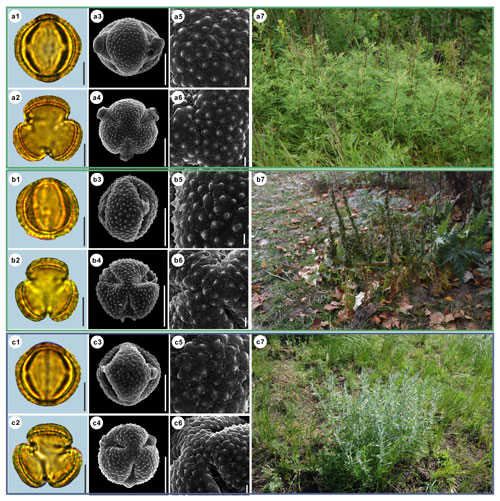
Figure 12Pollen grains and the habitats of their source plants. (a) Artemisia tanacetifolia; (b) Artemisia tournefortiana; and (c) Artemisia dracunculus. Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 cited from https://www.inaturalist.org/photos/78902853, last access: 19 August 2022, by © Alexander Dubynin, b7 provided by © Chen Chen, c7 cited from https://www.inaturalist.org/photos/76312868, last access: 19 August 2022, by © Anatoly Mikhaltsov). Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
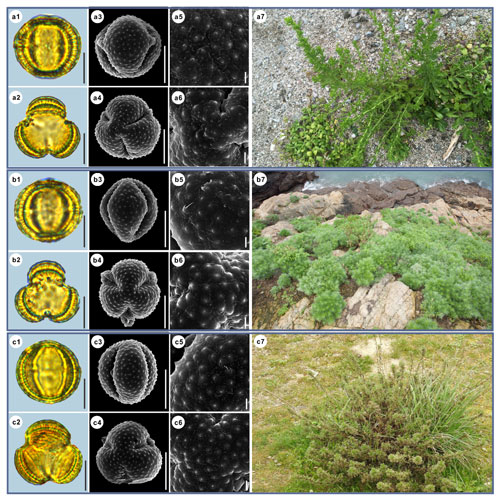
Figure 13Pollen grains and the habitats of their source plants.
(a) Artemisia japonica; (b) Artemisia capillaris; and (c) Artemisia campestris.
Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 cited from
https://www.inaturalist.org/photos/44507659, last access: 19 August 2022, by ©
![]()
![]()
![]() , b7 cited from https://www.inaturalist.org/photos/60639286, last access: 19 August 2022, by © Cheng-Tao Lin,
c7 cited from https://www.inaturalist.org/photos/113822257, last access: 19 August 2022, by
© pedrosanz-anapri).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
, b7 cited from https://www.inaturalist.org/photos/60639286, last access: 19 August 2022, by © Cheng-Tao Lin,
c7 cited from https://www.inaturalist.org/photos/113822257, last access: 19 August 2022, by
© pedrosanz-anapri).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
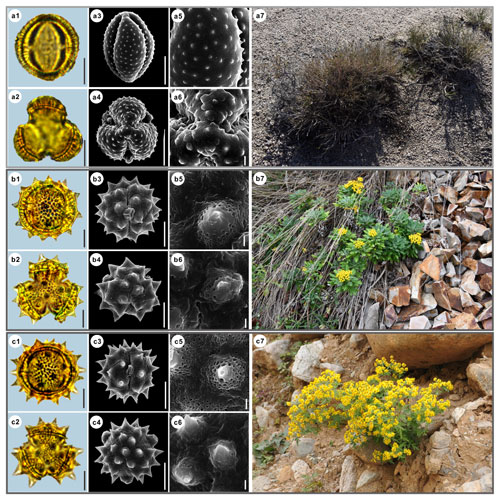
Figure 14Pollen grains and the habitats of their source plants.
(a) Kaschgaria brachanthemoides; (b) Ajania pallasiana; and (c) Chrysanthemum indicum.
Pollen grains in equatorial view under LM (a1, b1, c1) and SEM (a3, a5, b3, b5, c3, c5), in polar view under LM (a2, b2, c2) and SEM (a4, a6, b4, b6, c4, c6), along with the habitats of their source plants (a7 provided by
© Chen Chen, b7 cited from https://www.inaturalist.org/photos/162408714, last access: 19 August 2022, by © ![]() ,
c7 provided by © Bo-Han Jiao).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
,
c7 provided by © Bo-Han Jiao).
Scale bar in LM and SEM overview 10 µm, and in SEM close-up 1 µm.
3.2 Statistical pollen morphological trait data of 36 sampled taxa
The mean values of 10 pollen morphological traits of 36 sampled species are listed in Table 1, and these data distribution patterns are shown in boxplots (Fig. 15) in the form of variation (25 %–75 %) and further described in the form of mean value ± standard deviation (M ±SD, Appendix A).
Table 1Pollen morphological traits of 36 selected species (P: polar length; E: equatorial width; T: exine thickness; L: pollen length; D: diameter of spinule base; H: spinule height; Gs: granule spacing; Ss: spinule spacing; Ps: perforation spacing).
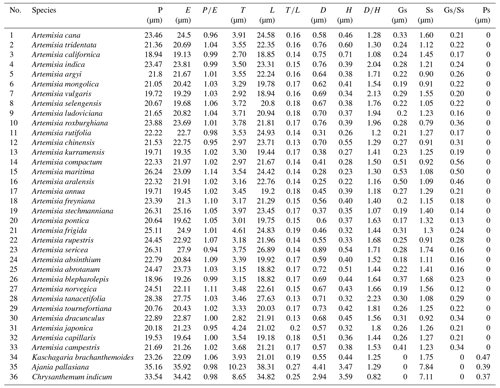
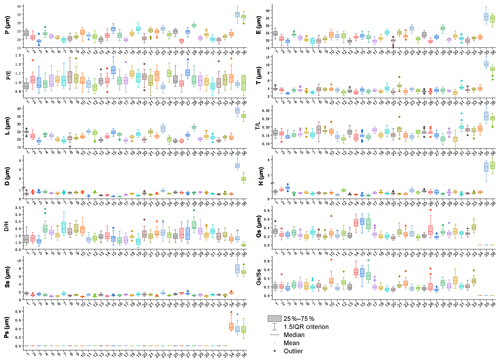
Figure 15Boxplots of 36 sampled taxa showing the variations in pollen morphological traits. 1. Artemisia cana; 2. Artemisia tridentata; 3. Artemisia californica; 4. Artemisia indica; 5. Artemisia argyi; 6. Artemisia mongolica; 7. Artemisia vulgaris; 8. Artemisia selengensis; 9. Artemisia ludoviciana; 10. Artemisia roxburghiana; 11. Artemisia rutifolia; 12. Artemisia chinensis; 13. Artemisia kurramensis; 14. Artemisia compactum; 15. Artemisia maritima; 16. Artemisia aralensis; 17. Artemisia annua; 18. Artemisia freyniana; 19. Artemisia stechmanniana; 20. Artemisia pontica; 21. Artemisia frigida; 22. Artemisia rupestris; 23. Artemisia sericea; 24. Artemisia absinthium; 25. Artemisia abrotanum; 26. Artemisia blepharolepis; 27. Artemisia norvegica; 28. Artemisia tanacetifolia; 29. Artemisia tournefortiana; 30. Artemisia dracunculus; 31. Artemisia japonica; 32. Artemisia capillaris; 33. Artemisia campestris; 34. Kaschagaria brachanthemoides; 35. Ajania pallasiana; 36. Chrysanthemum indicum.
3.3 The source plant occurrences
The source plant distributions in global terrestrial biomes of 36 sampled species are shown in Fig. 16. In Artemisia, some species have worldwide distributions, such as A. vulgaris (Fig. 16-7), A. absinthium (Fig. 16-24), and A. campestris (Fig. 16-33); a few taxa are limited to East Asia, such as A. roxburghiana (Fig. 16-10) and A. blepharolepis (Fig. 16-26), while others have narrow and isolated distributions in deserts and xeric shrublands of Central Asia, e.g. A. kurramensis (Fig. 16-13) and A. aralensis (Fig. 16-16). In outgroups of Artemisia, Kaschagaria brachanthemoides is also confined to deserts and xeric shrublands of Central Asia (Fig. 16-34), while Ajania pallasiana lives in forests of East Asia (Fig. 16-35).
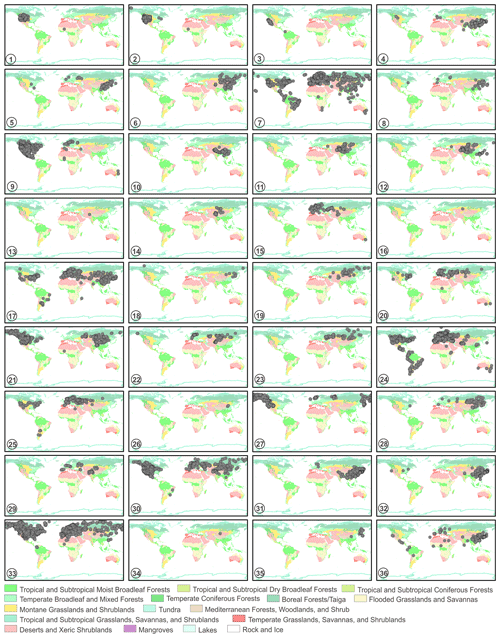
Figure 16The global distribution maps of 36 sampled taxa in terrestrial biomes (modified from Olson et al., 2001). 1. Artemisia cana; 2. Artemisia tridentata; 3. Artemisia californica; 4. Artemisia indica; 5. Artemisia argyi; 6. Artemisia mongolica; 7. Artemisia vulgaris; 8. Artemisia selengensis; 9. Artemisia ludoviciana; 10. Artemisia roxburghiana; 11. Artemisia rutifolia; 12. Artemisia chinensis; 13. Artemisia kurramensis; 14. Artemisia compactum; 15. Artemisia maritima; 16. Artemisia aralensis; 17. Artemisia annua; 18. Artemisia freyniana; 19. Artemisia stechmanniana; 20. Artemisia pontica; 21. Artemisia frigida; 22. Artemisia rupestris; 23. Artemisia sericea; 24. Artemisia absinthium; 25. Artemisia abrotanum; 26. Artemisia blepharolepis; 27. Artemisia norvegica; 28. Artemisia tanacetifolia; 29. Artemisia tournefortiana; 30. Artemisia dracunculus; 31. Artemisia japonica; 32. Artemisia capillaris; 33. Artemisia campestris; 34. Kaschagaria brachanthemoides; 35. Ajania pallasiana; 36. Chrysanthemum indicum.
4.1 The pollen classification of Artemisia
The pollen grains of Anthemideae and Asteraceae under LM could be simply divided into Artemisia pollen type (Figs. 3–13 and 14a, Appendix A) with indistinct and short spinules, and Anthemis pollen type such as Chrysanthemum indicum and Ajania pallasiana (Fig. 14b and c, Appendix A) with distinct and long spines on pollen exine ornamentation (Wodehouse, 1926; Stix, 1960; Chen, 1987; Chen and Zhang, 1991; Martín et al., 2001, 2003; Sanz et al., 2008; Blackmore et al., 2009; Vallès et al., 2011). Artemisia pollen grains are difficult to separate from those of other related genera with Artemisia pollen type such as Kaschgaria brachanthemoides (Fig. 14a1 and a2, Appendix A), Elachanthemum, Ajaniopsis, Filifolium, and Neopallasia (Chen and Zhang, 1991) under LM due to their great similarity in pollen exine ornamentation and colporate patterns (Chen, 1987; Martín et al., 2001, 2003; Vallès et al., 2011). Furthermore, Sing and Joshi (1969) questioned the feasibility of recognizing pollen types under LM in the highly uniform pollen of Artemisia. Later, SEM made it possible to subdivide the pollen of Artemisia and those of other related genera within the Artemisia pollen type using pollen exine ultrastructure characters (Chen, 1987; Chen and Zhang, 1991; Sun and Xu, 1997; Jiang et al., 2005; Ghahraman et al., 2007; Shan et al., 2007; Hayat et al., 2009, 2010; Hussain et al., 2019).
Hierarchical cluster analysis (Fig. 17a) revealed that the pollen morphological traits (, H, D, , Ss, Gs, , and Ps) of Artemisia and its outgroups were divided into Clade A with perforations and without granules (Fig. 13a5 and a6, b5 and b6, c5 and c6), and Clade B with granules and without perforations (Figs. 3–13a5 and a6, b5 and b6, c5 and c6) on the pollen exine under SEM.
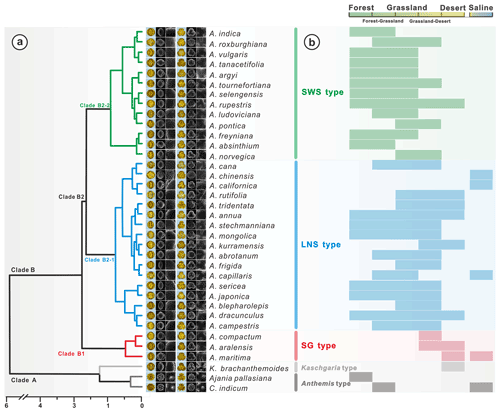
Figure 17Hierarchical cluster analysis, showing the dendrogram for pollen types from Artemisia and outgroups (a), and the habitat ranges of 36 representative species (b, Tutin et al., 1976; Zhang, 2007; Ling et al., 2011).
In addition, Clade A, as the outgroup of Artemisia, includes Anthemis type (Chrysanthemum indicum and Ajania pallasiana) with prominent spines on pollen exine under LM, and Kaschgaria type (Kaschgaria brachanthemoides) with spinules on pollen exine (Figs. 14a and 17a). Clade B comprises three pollen types from three branches of Artemisia (Fig. 17a), i.e. SG type (short and wide spinule pollen type, Clade B1), LNS type (long and narrow spinule pollen type, Clade B2-1), and SG type (sparse granule pollen type, Clade B2-2).
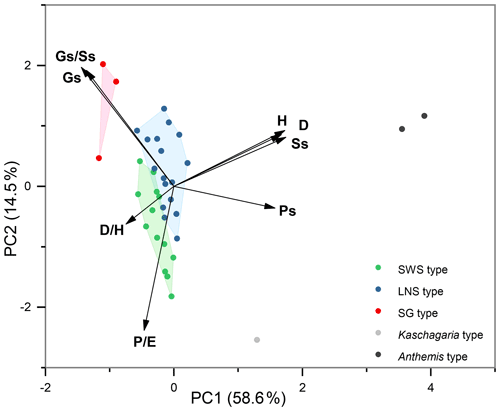
Eight pollen morphological traits (, H, D, , Ss, Gs, , and Ps) were selected for the principal component analysis (PCA) of 36 taxa of Artemisia and its outgroups (Fig. 18), and grouped according to the five clades of the cluster analysis, i.e. the five pollen types (Fig. 17a). The results reveal that Artemisia pollen morphology differs significantly from that of the outgroups, and that three Artemisia pollen types could be distinguished.
Nine characteristics of Artemisia pollen could partially explain the differences between these three pollen types (Fig. 19). (the length of polar axis/the length of equatorial axis) in LNS types (0.93–1.06) are significantly different (ANOVA p<0.001) from both SWS (0.97–1.12) and SG (0.98–1.14), so could be used to identify the LNS type. (diameter of spinule base/spinule height) in the SWS type differ significantly (ANOVA p<0.001) from both LNS and SG types. The variation range of is 1.38–2.23 in the SWS type, 1.07–1.75 in the LNS type, and 0.98–1.66 in the SG type, indicating that the SWS pollen type is distinguished by short and wide spinules. (granule spacing/spinule spacing) in the SG type was higher than those of the SWS and LNS types (ANOVA p<0.001), which distinguished the SG type from the other two types. Moreover, the SG type is characterized by sparse granules with the variation range of spanning 0.37–0.64, while the SWS and LNS types show much denser granules whose are mainly below 0.35.
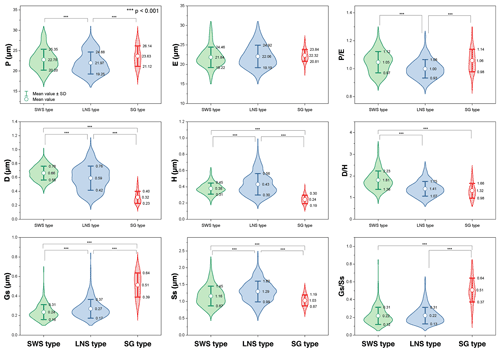
Figure 19Violin diagrams of three pollen types from Artemisia, showing the variations (M ±SD) in nine pollen characters (P: length of polar axis; E: length of equatorial axis; D: diameter of spinule base; H: spinule height; Gs: granule spacing; Ss: spinule spacing; Ps: perforation spacing). Asterisks indicate statistically significant differences (p<0.001).
Within the new Artemisia pollen classification (Fig. 17a, Key), the SWS type represents a type of pollen with short and wide spinules () and dense granules (Figs. 17a and 19). The LNS type represents a type of pollen with long and narrow spinules () and dense granules (Figs. 17a and 19). The SG type is characterized by sparse granules () and small, long, and narrow spinules (Figs. 17a and 19).
4.2 Testing the pollen intraspecific variability within Artemisia
Evidence shows that the pollen morphology in Artemisia is highly uniform under LM without discrimination (Wodehouse, 1926; Sing and Joshi, 1969; Ling, 1982; Chen, 1987; Wang et al., 1995), which might suggest that statistical analyses of the intraspecific morphological variation of pollen under the LM are limited or meaningless. Right now, the SEM technique has made it possible to subdivide Artemisia pollen into different types using pollen exine ultrastructure characters (Chen, 1987; Chen and Zhang, 1991; Sun and Xu, 1997; Jiang et al., 2005; Ghahraman et al., 2007; Shan et al., 2007; Hayat et al., 2009, 2010; Hussain et al., 2019).
In order to test the intraspecific variability of pollen exine ultrastructure traits, we selected one species respectively from the three pollen types corresponding to the three morphological clades of Artemisia pollen, i.e. Artemisia vulgaris (SWS type), Artemisia annua (LNS type), and Artemisia maritima (SG type), and sampled five specimens of each species (Table B2). Six pollen traits, i.e. D, H, , Gs, Ss, and , were counted and analysed under SEM to test for intraspecific variability of pollen exine ultrastructure traits.
The test showed that it was feasible to use stable and for pollen type classification of Artemisia because (1) and were stable within species (Fig. 20 and Table 2) for the pollen classification; and (2) D, H, Gs, and Ss were variable as size values, e.g. these four traits were significantly different within species in both A. vulgaris and A. annua, while D, H, and Ss were significantly different within species in A. maritima (Fig. 20 and Table 2). There was evidence showing that size values such as pollen exine ultrastructure size were often variable within species due to their genetic divergence, various habitats, and different experimental treatments (Mo et al., 1997; Zhao and Yao, 1999; Zhang and Qian, 2011).
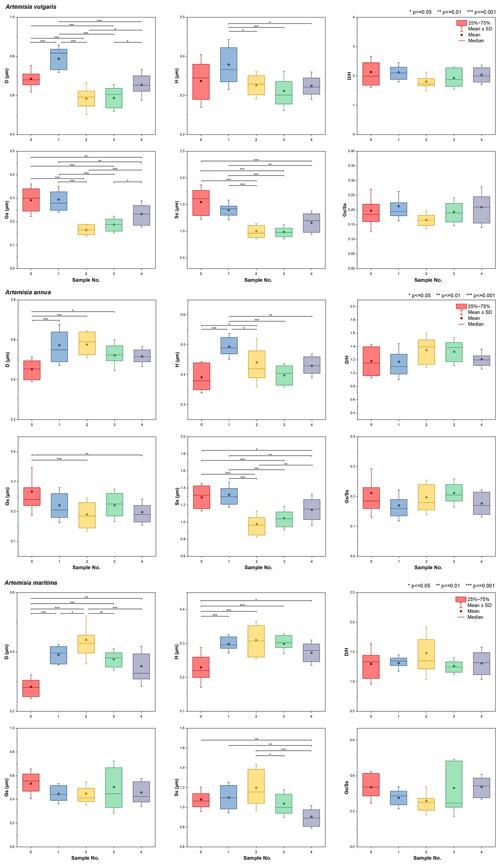
Figure 20Boxplots of intraspecific pollen exine ultrastructure characters from three species of Artemisia, showing the variations (M ±SD) in six pollen characters (D: diameter of spinule base; H: spinule height; Gs: granule spacing; Ss: spinule spacing; Ps: perforation spacing). Asterisks indicate statistically significant differences (∗ p≤0.05, p≤0.01, p<0.001).
Table 2The results of ANOVA for intraspecific variability in pollen exine ultrastructure characters among three representative species.
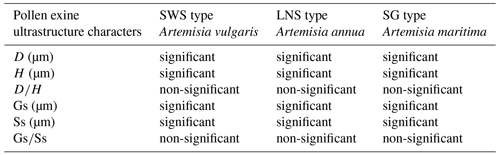
Key to three pollen types of Artemisia and two pollen types of its outgroups
- 1.
Pollen exine with perforations and without granules under SEM 2
- 1.
Pollen exine with granules and without perforations under SEM 3
- 2.
Distinct and long spines on pollen exine, with H>3 µm Anthemis type
- 2.
Indistinct and short spinules on pollen exine, with H<1 µm Kaschgaria type
- 3.
Pollen exine with sparse granules and under SEM SG type
- 3.
Pollen exine with dense granules and under SEM 4
- 4.
Pollen exine with under SEM LNS type
- 4.
Pollen exine with under SEM SWS type
4.3 The ecological implications of Artemisia pollen types
Plotting the distribution data of 33 species from nine main branches of Artemisia constrained by the phylogenetic framework (Fig. 1) onto the global terrestrial biomes (Fig. 21), we noticed that the genus is widely distributed from forest to grassland, desert, and saline habitats (Figs. 16, 17b, and 21). Furthermore, different species of Artemisia with SWS pollen type (Fig. 21a) and LNS type (Fig. 21b) have a rather wide distribution with severely overlapping ranges, while those with SG type (Fig. 21c) have narrow and isolated distributions.
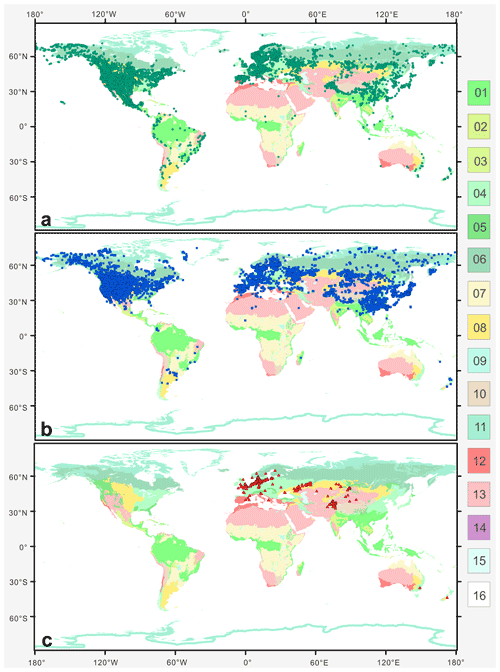
Figure 21The global distribution pattern of three Artemisia pollen types in terrestrial biomes (modified from Olson et al., 2001). (a) SG type; (b) LNS type; and (c) SWS type. Fourteen terrestrial biomes: 01. tropical and subtropical moist broadleaf forests; 02. tropical and subtropical dry broadleaf forests; 03. tropical and subtropical coniferous forests; 04. temperate broadleaf and mixed forests; 05. temperate coniferous forests; 06. boreal forests/taiga; 07. flooded grasslands and savannas; 08. montane grasslands and shrublands; 09. tundra; 10: Mediterranean forests, woodlands, and shrub; 11. tropical and subtropical grasslands, savannas, and shrublands; 12. temperate grasslands, savannas, and shrublands; 13. deserts and xeric shrublands; 14. mangroves; 15. lakes; 16. rock and ice.
The ecological implications of Artemisia pollen types mentioned above fall into four categories. (i) Artemisia with the SG pollen type all belong to the subg. Seriphidium, which generally grows in dry habitats ranging from grassland desert to desert and coastal saline–alkaline environments, with their distribution largely limited to Eurasia and growing at low altitude (Figs. 17b, 21c, and 22). (ii) The habitats of Artemisia with LNS pollen type have a global distribution and occur in forest, grassland and desert, and even coastal areas (Figs. 17b, 21b, and 22), with the highest mean annual temperature (MAT). Hence, the LNS pollen type is a generalist. (iii) Artemisia with SWS pollen type include Sect. Artemisia, and its habitats range from forest to desert, although most of the taxa are confined to humid environments from forest to grassland with a global distribution and the highest mean annual precipitation (MAP, Figs. 17b, 21c, and 22). (iv) If the SWS pollen type and the SG pollen type appear together, the range of vegetation types could be reduced to grassland desert and desert through niche coexistence (Fig. 17b).
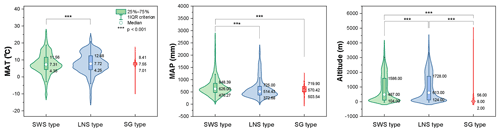
Figure 22Violin diagrams of three pollen types from Artemisia, showing the variations (25 %–75 %) in MAT, MAP, and altitude. Asterisks indicate statistically significant differences (p<0.001).
In addition, we noticed that Kaschgaria brachanthemoides as an outgroup of Artemisia lives in dry mountain valleys or dry riverbeds of northwestern China (Toksun) and Kazakhstan, with highly characteristic pollen (Fig. 14a), narrow habitats (Fig. 17b), and regional distribution (Fig. 16-34), and has the potential to indicate some specific habitats.
Pollen datasets (Table 3) including pollen photographs under LM and SEM, statistical data of pollen morphological traits, and their source plant distribution for each species are available at Zenodo (https://doi.org/10.5281/zenodo.6900308; Lu et al., 2022).
To cover the maximum range of Artemisia pollen morphological variation, we provide a pollen dataset of 36 species from nine clades and three outgroups of Artemisia constrained by the phylogenetic framework, containing high-quality pollen photographs under LM and SEM, statistical data of pollen morphological traits together with their source plant distribution, and corresponding environmental factors. Here, we attempt to decipher the underlying causes of the long-standing disagreement in the palynological community on the correlation between Artemisia pollen and aridity by recognizing the different ecological implications of Artemisia pollen types.
This dataset should work well for identifying and classifying Artemisia pollen from Neogene and Quaternary sediments. While Artemisia pollen grains are uniform in morphology under LM, different types can be recognized under SEM. So, the single-grain technique for picking out fossil pollen grains and photographing the same grains under LM and SEM should provide valuable insights in the diversity of fossil Artemisia (Ferguson et al., 2007; Grímsson et al., 2011, 2012; Halbritter et al., 2018). Furthermore, those Artemisia pollen grains could then be compared with the rich photographs from this dataset, and together with the key provided here, possibly attributed to one of the three Artemisia pollen types, which in turn may provide a link to the different habitat ranges.
However, the application of this dataset probably may not work well for the Palaeogene, as (1) Artemisia might have originated in the Palaeocene, although there is no evidence for a specific location or time interval of its origin (e.g. Ling, 1982; Wang, 2004; Miao et al., 2011); (2) both the lack of macrofossils of Artemisia and the strong pollen similarity between Artemisia and its closely related taxa under LM might lead to confusion and more uncertainty in tracing the origin of Artemisia. On the other hand, the present dataset provides a potential morphological tool to distinguish Artemisia pollen grains from those of its related taxa at the SEM level and may shed light on the origin of this genus in the Palaeogene.
Moreover, these pollen photographs also have potential and the possibility to be used for deep learning research. We are attempting to automatically identify pollen images using pollen assemblages from the eastern Central Asian desert as an example with deep convolutional neural network (DCNN) of artificial intelligence. Pollen images of the many species of Artemisia provided here, and the increasing number of intraspecific replications in the future, will all serve for projected image identification research.
Finally and most importantly, the Artemisia pollen dataset as designed is open and expandable for new pollen data from Artemisia worldwide in order to better serve the global environment assessment and refined reconstruction of vegetation in the geological past as a basis or blueprint for other overarching statistical analyses on pollen morphology.
Pollen morphological descriptions of 36 representative species from nine clades of Artemisia and three outgroups.
Pollen morphology of Artemisia: pollen grains oblate, spherical, or ellipsoidal; apertures tricolporate; almost circular in equatorial view and trilobate circular in polar view; the exine near the colpi gradually thinned; the exine has an obvious double structure of inner and outer layers where the outer is thicker than the inner under LM; and the exine ornamentation is psilate (LM), spinulate, and granule (SEM).
-
Artemisia cana (Table 1, Figs. 3a and 15).
Pollen grains spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. Polar length µm (M ±SD), equatorial width µm (M ±SD), (M ±SD), exine thickness µm (M ±SD), and pollen length µm (M ±SD), . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, diameter of spinule base µm (M ±SD), spinule height µm (M ±SD), (M ±SD), granule spacing µm (M ±SD), and spinule spacing µm (M ±SD), (M ±SD).
Habitat: grasslands, gravel soils, mountain meadows, and stream banks; wet mountain meadows, stream banks, and rocky areas with late-lying snows.
-
Artemisia tridentata (Table 1, Figs. 3b and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: mountains, grasslands, and meadows of western North America. Arid and semi-arid, desert, or semi-desert areas of the growing shrub or semi-shrub environment.
-
Artemisia californica (Table 1, Figs. 3c and 15).
Pollen grains prolate or spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: coastal scrub and dry foothills.
-
Artemisia indica (Table 1, Figs. 4a and 15).
Pollen grains spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: roadsides, forest margins, slopes, and shrublands; low elevations to 2000 m.
-
Artemisia argyi (Table 1, Figs. 4b and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: waste places, roadsides, slopes, hills, steppes, and forest steppes; low elevations to 1500 m.
-
Artemisia mongolica (Table 1, Figs. 4c and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: slopes, shrublands, riverbanks, lakeshores, roadsides, steppes, forest steppes, and dry valleys; low elevations to 2000 m.
-
Artemisia vulgaris (Table 1, Figs. 5a and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: roadsides, slopes, canyons, forest margins, forest steppes, and subalpine steppes; 1500–3800 m.
-
Artemisia selengensis (Table 1, Figs. 5b and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: riverbanks, lakeshores, humid areas, meadows, slopes, and roadsides.
-
Artemisia ludoviciana (Table 1, Figs. 5c and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: disturbed roadsides, open meadows, and rocky slopes.
-
Artemisia roxburghiana (Table 1, Figs. 6a and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: roadsides, slopes, dry canyons, grasslands, waste areas, and terraces; 700–3900 m.
-
Artemisia rutifolia (Table 1, Figs. 6b and 15).
Pollen grains spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: hills, dry river valleys, basins, steppes, semideserts, and stony desert; 1300–5000 m.
-
Artemisia chinensis (Table 1, Figs. 6c and 15).
Pollen grains spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: littoral plants found on raised coral outcrops.
-
Artemisia kurramensis (Table 1, Figs. 7a and 15).
Pollen grains spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: foothills, mountain slopes, dry graveyards, and field borders with sparse vegetation on gravelly, fine to coarse sandy-clay soils.
-
Artemisia compactum (Table 1, Figs. 7b and 15).
Pollen grains spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: rocky slopes and semi-deserts, from low elevations to sub-alpine areas.
-
Artemisia maritima (Table 1, Figs. 7c and 15).
Pollen grains prolate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: saltmarsh, dry and calcareous hillsides, seashores, and dry saline or alkaline soils.
-
Artemisia aralensis (Table 1, Figs. 8a and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: clayey, sandy loam, and solonetzic soils.
-
Artemisia annua (Table 1, Figs. 8b and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: hills, waysides, wastelands, outer forest margins, steppes, forest steppes, dry flood lands, terraces, semidesert steppes, rocky slopes, roadsides, and saline soils; 2000–3700 m.
-
Artemisia freyniana (Table 1, Figs. 8c and 15).
Pollen grains prolate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: steppes, slopes, dry river valleys, riverbanks, and outer forest margins.
-
Artemisia stechmanniana (Table 1, Figs. 9a and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: hillsides, roadsides, shrubland, and forest-steppe areas, and often becoming the dominant species or main associated species of plant communities in some areas of mountainous sunny slopes.
-
Artemisia pontica (Table 1, Figs. 9b and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: rocky slopes, dry valleys, steppes, and hills; low to middle elevations.
-
Artemisia frigida (Table 1, Figs. 9c and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: steppes, sub-alpine meadows, dry hillsides, stable dunes, and dry waste areas; 1000–4000 m.
-
Artemisia rupestris (Table 1, Figs. 10a and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: dry hills, desert or semidesert steppes, grassy marshlands, dry river valleys, riverbeds, scrub, and forest margins.
-
Artemisia sericea (Table 1, Figs. 10b and 15).
Pollen grains spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: forest margins, hills, steppes, canyons, and waste areas.
-
Artemisia absinthium (Table 1, Figs. 10c and 15).
Pollen grains prolate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: hillsides, steppes, scrub, forest margins, and often in locally moist situations; 1100–1500 m.
-
Artemisia abrotanum (Table 1, Figs. 11a and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: the wasteland of western, southern, central, and southern Europe.
-
Artemisia blepharolepis (Table 1, Figs. 11b and 15).
Pollen grains spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: low-altitude areas of dry slopes, grasslands, steppes, waste areas, roadsides, and dunes near riverbanks.
-
Artemisia norvegica (Table 1, Figs. 11c and 15).
Pollen grains prolate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: bare stony ground, Racomitrium heath, bouldery crests of solifluction terraces, and sometimes hollows between rocks.
-
Artemisia tanacetifolia (Table 1, Figs. 12a and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: middle and low-altitude areas of forest grasslands, grasslands, meadows, forest edges, open forests, salty grasslands, grass slopes, and brushwood.
-
Artemisia tournefortiana (Table 1, Figs. 12b and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: widely distributed on hills, terraces, dry flood lands, waste fields, steppes, open forests, and semi-marshlands.
-
Artemisia dracunculus (Table 1, Figs. 12c and 15).
Pollen grains spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: dry slopes, steppes, semidesert steppes, forest steppes, forest margins, waste areas, roadsides, terraces, subalpine meadows, meadow steppes, dry river valleys, rocky slopes, and saline–alkaline soils; 500–3800 m.
-
Artemisia japonica (Table 1, Figs. 13a and 15).
Pollen grains spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: forest margins, waste areas, shrublands, hills, slopes, and roadsides. Low elevations to 3300 m.
-
Artemisia capillaris (Table 1, Figs. 13b and 15).
Pollen grains spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: humid slopes, hills, terraces, roadsides, and riverbanks; 100–2700 m.
-
Artemisia campestris (Table 1, Figs. 13c and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , µm, µm, .
Habitat: steppes, waste areas, rocky slopes, and dune margins; 300–3100 m.
-
Kaschgaria brachanthemoides (Table 1, Figs. 14a and 15).
Pollen grains prolate or spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation is psilate (LM), spinulate (SEM). Under SEM, µm, µm, , Gs=0 µm, µm, , perforations spacing µm.
Habitat: dry mountain valleys, and old dry riverbeds; 1000–1500 m.
-
Ajania pallasiana (Table 1, Figs. 14b and 15).
Pollen grains spheroidal. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, µm. The exine ornamentation spinose. Under SEM, µm, µm, , Gs=0 µm, µm, , µm.
Habitat: thickets and mountain slopes; 200–2900 m.
-
Chrysanthemum indicum (Table 1, Figs. 14c and 15).
Pollen grains prolate or spheroidal or oblate. Almost circular in equatorial view and trilobate circular in polar view. Apertures tricolporate. The exine near the colpi gradually thinned. µm, µm, , µm, µm, . The exine ornamentation spinose. Under SEM, µm, µm, , Gs=0 µm, µm, , µm.
Habitat: grasslands on mountain slopes, thickets, wet places by rivers, fields, roadsides, saline places by seashores, and under shrubs; 100–2900 m.
Table B1List of the voucher specimen in PE Herbarium, Institute of Botany, Chinese Academy of Sciences.
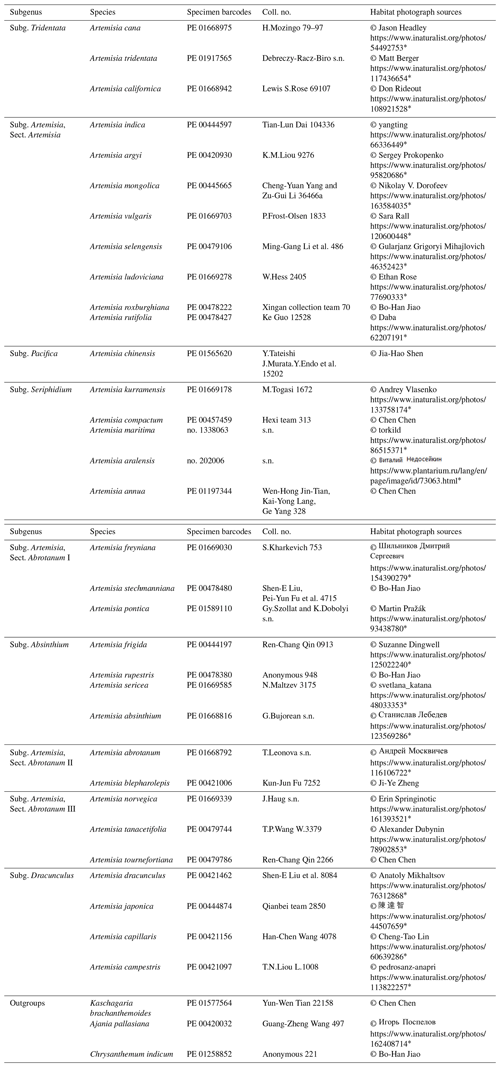
Note: in the absence of habitat photographs of two species, habitat photographs of species with which they have close phylogenetic relationships and similar habitats were used in this study instead, i.e. the habitat photograph of Kaschagaria komarovii was used instead of Kaschagaria brachanthemoides, and the habitat photograph of Artemisia taurica for Artemisia kurramensis. * Last access: 19 August 2022
YFW, YFY, and TGG conceived the ideas; LLL, BHJ, KQL, and BS collected the literature; LLL extracted and compiled the data, LLL, FQ, and BHJ made the statistical analysis; GX and ML collected pictures; LLL, KQL, and BS drew the figures and tables; LLL, YFW, YFY, JFL, FQ, and GX wrote the first draft of this article; DKF corrected the various versions of the article; and all authors contributed substantially to revisions.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We thank Jian Yang, Institute of Botany, Chinese
Academy of Sciences, for his kind help in drafting graphics. We appreciate
Chen Chen from Institute of Botany, Chinese Academy of Sciences, Ji-Ye Zheng
from No. 1 Middle School of Jiyang Shandong, Jia-Hao Shen from Institute
of Botany, Jiangsu Province and Chinese Academy of Sciences (Nanjing
Botanical Garden Mem. Sun Yat-Sen), and ![]()
![]() from Almaty, Kazakhstan, for their enthusiastic assistance in
providing habitat photographs.
from Almaty, Kazakhstan, for their enthusiastic assistance in
providing habitat photographs.
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB26000000), National Natural Science Foundation of China (grant nos. 31970233, 32070240, 31870179, 31570204, 42077416, 32000174 and 42077423), the Chinese Academy of Sciences President’s International Fellowship Initiative (grant no. 2018VBA0016), the Sino-Africa Joint Research Center (grant no. SAJC201614), Key Project at Central Government Level: the ability establishment of sustainable use for valuable Chinese medicine resources (grant no. 2060302), National Plant Specimen Resource Bank (grant no. E0117G1001), and the International Partnership Program of Chinese Academy of Sciences (grant no. 151853KYSB20190027).
This paper was edited by Alessio Rovere and reviewed by Angela Bruch and one anonymous referee.
Beerling, D. J. and Royer, D. L.: Convergent Cenozoic CO2 history, Nat. Geosci., 4, 418–420, https://doi.org/10.1038/ngeo1186, 2011.
Bhattacharya, T., Tierney, J. E., Addison, J. A., and Murray, J. W.: Ice-sheet modulation of deglacial North American monsoon intensification, Nat. Geosci., 11, 848–852, https://doi.org/10.1038/s41561-018-0220-7, 2018.
Blackmore, S., Wortley, A. H., Skvarla, J. J., and Robinson, H.: Evolution of pollen in Compositae, in: Systematics, Evolution and Biogeography of the Compositae, edited by: Funk, V. A., Susanna, A., Stuessy, T. F., and Bayer, R. J., International Association of Plant Taxonomy, Vienna, 102–130, ISBN 978-3-9501754-3-1 2009.
Bremer, K. and Humphries, C. J.: Generic monograph of the Asteraceae-Anthemideae, Bull. Nat. Hist. Mus., 23, 71–177, https://www.biodiversitylibrary.org/item/19562 (last access: 19 August 2022), 1993.
Brummitt, N., Araujo, A. C., and Harris, T.: Areas of plant diversity – What do we know?, Plants People Planet, 3, 33–44, https://doi.org/10.1002/ppp3.10110, 2021.
Cai, M., Ye, P., Yang, X., and Li, C.: Vegetation and climate change in the Hetao Basin (Northern China) during the last interglacial-glacial cycle, J. Asian Earth Sci., 171, 1–8, https://doi.org/10.1016/j.jseaes.2018.11.024, 2019.
Cao, X., Tian, F., Li, K., Ni, J., Yu, X., Liu, L., and Wang, N.: Lake surface sediment pollen dataset for the alpine meadow vegetation type from the eastern Tibetan Plateau and its potential in past climate reconstructions, Earth Syst. Sci. Data, 13, 3525–3537, https://doi.org/10.5194/essd-13-3525-2021, 2021.
Chen, S. B.: Study on the pollen morphology of the Chinese genus Artemisia L. and the relationship with its allies, Institute of Botany, Chinese Academy of Sciences, Beijing, China, http://ir.ibcas.ac.cn/handle/2S10CLM1/12089 (last access: 19 August 2022), 1987 (in Chinese).
Chen, S. B. and Zhang, J. T.: A Study on pollen morphology of some Chinese Genera in tribe Anthemideae, Acta Phytotaxon. Sin., 29, 246–251, 1991 (in Chinese).
Cui, Q. Y., Zhao, Y., Qin, F., Liang, C., Li, Q., and Geng, R. W.: Characteristics of the modern pollen assemblages from different vegetation zones in Northeast China: Implications for pollen-based climate reconstruction, Sci. China Earth Sci., 62, 1564–1577, https://doi.org/10.1007/s11430-018-9386-9, 2019.
Davies, C. P. and Fall, P. L.: Modern pollen precipitation from an elevational transect in central Jordan and its relationship to vegetation, J. Biogeogr., 28, 1195–1210, https://doi.org/10.1046/j.1365-2699.2001.00630.x, 2001.
El-Moslimany, A. P.: Ecological significance of common nonarboreal pollen: examples from drylands of the Middle East, Rev. Palaeobot. Palyno., 64, 343–350, https://doi.org/10.1016/0034-6667(90)90150-h, 1990.
Erdtman, G.: The acetolysis method, a revised descriptions, Svensk Botanisk Tidskrift, 54, 561–564, 1960.
Ferguson, D. K., Zetter, R., and Paudayal, K. N.: The need for the SEM in palaeopalynology, C. R. Palevol, 6, 423–430, https://doi.org/10.1016/j.crpv.2007.09.018, 2007.
GBIF.org: GBIF Occurrence Download, https://doi.org/10.15468/dl.596xd9, last access: 9 November 2021.
Ghahraman, A., Nourbakhsh, N., Mehdi, G. K., and Atar, F.: Pollen morphology of Artemisia L. (Asteraceae) in Iran, Iran. J. Bot., 13, 21–29, 2007.
Grímsson, F., Zetter, R., and Hofmann, C.: Lythrum and Peplis from the Late Cretaceous and Cenozoic of North America and Eurasia: new evidence suggesting early diversification within the Lythraceae, Am. J. Bot., 98, 1801–1815, https://doi.org/10.3732/ajb.1100204, 2011.
Grímsson, F., Zetter, R., and Leng, Q.: Diverse fossil Onagraceae pollen from a Miocene palynoflora of north-east China: early steps in resolving the phytogeographic history of the family, Plant Syst. Evol., 298, 671–687, https://doi.org/10.1007/s00606-011-0578-0, 2012.
Guiot, J. and Cramer, W.: Climate change: The 2015 Paris Agreement thresholds and Mediterranean basin ecosystems, Science, 354, 465–468, https://doi.org/10.1126/science.aah5015, 2016.
Halbritter, H., Silvia, U., Grímsson, F., Weber, M., Zetter, R., Hesse, M., Buchner, R., Svojtka, M., and Frosch-Radivo, A.: Illustrated pollen terminology, Springer Open, https://doi.org/10.1007/978-3-319-71365-6, 2018.
Hayat, M. Q., Ashraf, M., Khan, M. A., Yasmin, G., Shaheen, N., and Jabeen, S.: Phylogenetic analysis of Artemisia L. (Asteraceae) based on micromorphological traits of pollen grains, Afr. J. Biotechnol., 8, 6561–6568, https://doi.org/10.1556/AMicr.56.2009.4.11, 2009.
Hayat, M. Q., Ashraf, M., Khan, M. A., Yasmin, G., and Jabeen, S.: Palynological study of the genus Artemisia (Asteraceae) and its systematic implications, Pak. J. Bot., 42, 751–763, https://doi.org/10.1094/MPMI-23-4-0522, 2010.
Herzschuh, U., Tarasov, P., Wünnemann, B., and Kai, H.: Holocene vegetation and climate of the Alashan Plateau, NW China, reconstructed from pollen data, Palaeogeogr. Palaeocl., 211, 1–17, https://doi.org/10.1016/j.palaeo.2004.04.001, 2004.
Hesse, M., Buchner, R., Froschradivo, A., Halbritter, H., Ulrich, S., Weber, M., and Zetter, R.: Pollen Terminology: An illustrated handbook, Springer, New York, https://doi.org/10.1093/aob/mcp289, 2009.
Hussain, A., Potter, D., Hayat, M. Q., Sahreen, S., and Bokhari, S. A. I.: Pollen morphology and its systematic implication on some species of Artemisia L. from Gilgit-Baltistan Pakistan, Bangl. J. Plant Taxon., 26, 157–168, https://doi.org/10.3329/bjpt.v26i2.44576, 2019.
Jiang, L., Q., W., Ye, L. Z., and R., and Ling, Y. R.: Pollen morphology of Artemisia L. and its systematic significance, Wuhan Univ. J. Nat. Sci., 10, 448–454, https://doi.org/10.1007/BF02830685, 2005.
Jinxia, C., Xuefa, S., Yanguang, L., Shuqing, Q., Shixiong, Y., Shijuan, Y., Huahua, L., Jianyong, L., Xiaoyan, L., and Chaoxin, L.: Holocene vegetation dynamics in response to climate change and hydrological processes in the Bohai region, Clim. Past, 16, 2509–2531, https://doi.org/10.5194/cp-16-2509-2020, 2020.
Koutsodendris, A., Allstadt, F. J., Kern, O. A., Kousis, I., Schwarz, F., Vannacci, M., Woutersen, A., Appel, E., Berke, M. A., Fang, X. M., Friedrich, O., Hoorn, C., Salzmann, U., and Pross, J.: Late Pliocene vegetation turnover on the NE Tibetan Plateau (Central Asia) triggered by early Northern Hemisphere glaciation, Global Planet. Change, 180, 117–125, https://doi.org/10.1016/j.gloplacha.2019.06.001, 2019.
Li, F., Sun, J., Zhao, Y., Guo, X., Zhao, W., and Zhang, K.: Ecological significance of common pollen ratios: A review, Front. Earth Sci.-PRC, 4, 253–258, https://doi.org/10.1007/s11707-010-0112-7, 2010.
Li, X. L., Hao, Q. Z., Wei, M. J., Andreev, A. A., Wang, J. P., Tian, Y. Y., Li, X. L., Cai, M. T., Hu, J. M., and Shi, W.: Phased uplift of the northeastern Tibetan Plateau inferred from a pollen record from Yinchuan Basin, northwestern China, Sci. Rep.-UK, 7, 10, https://doi.org/10.1038/s41598-017-16915-z, 2017.
Ling, Y. R.: On the system of the genus Artemisia Linn. and the relationship with allies, Bulletin of Botanical Research, 2, 1–60, 1982 (in Chinese).
Ling, Y. R., Humphries, C. J., and Gilbert, M. G.: Artemisia L., in: Flora of China Volume 20–21 (Asteraceae), edited by: Wu, Z. Y., Raven, P. H., and Hong, D. Y., Science Press, Beijing & Missouri Botanical Garden Press, St. Louis, 678–739, ISBN 9781935641094, 2011.
Liu, H. Y., Wang, Y., Tian, Y. H., Zhu, J. L., and Wang, H. Y.: Climatic and anthropogenic control of surface pollen assemblages in East Asian steppes, Rev. Palaeobot. Palyno., 138, 281–289, https://doi.org/10.1016/j.revpalbo.2006.01.008, 2006.
Lu, K. Q., Qin, F., Li, Y., Xie, G., Li, J. F., Cui, Y. M., Ferguson, D. K., Yao, Y. F., Wang, G. H., and Wang, Y. F.: A new approach to interpret vegetation and ecosystem changes through time by establishing a correlation between surface pollen and vegetation types in the eastern central Asian desert, Palaeogeogr. Palaeocl., 551, 12, https://doi.org/10.1016/j.palaeo.2020.109762, 2020.
Lu, L. L, Jiao, B. H., Qin, F., Xie, G., Lu, K. Q., Li, J. F., Sun, B., Li, M., Ferguson, D. K., Gao, T. G., Yao, Y. F., and Wang, Y. F.: Artemisia pollen dataset for exploring the potential ecological indicators in deep time, Zenodo [data set], https://doi.org/10.5281/zenodo.6900308, 2022.
Ma, Q. F., Zhu, L. P., Wang, J. B., Ju, J. T., Lu, X. M., Wang, Y., Guo, Y., Yang, R. M., Kasper, T., Haberzettl, T., and Tang, L. Y.: ArtemisiaChenopodiaceae ratio from surface lake sediments on the central and western Tibetan Plateau and its application, Palaeogeogr. Palaeocl., 479, 138–145, https://doi.org/10.1016/j.palaeo.2017.05.002, 2017.
Malik, S., Vitales, D., Hayat, M. Q., Korobkov, A. A., Garnatje, T., and Valles, J.: Phylogeny and biogeography of Artemisia subgenus Seriphidium (Asteraceae: Anthemideae), Taxon, 66, 934–952, https://doi.org/10.12705/664.8, 2017.
Marsicek, J., Shuman, B. N., Bartlein, P. J., Shafer, S. L., and Brewer, S.: Reconciling divergent trends and millennial variations in Holocene temperatures, Nature, 554, 92–96, https://doi.org/10.1038/nature25464, 2018.
Martín, J., Torrell, M., and Valles, J.: Palynological features as a systematic marker in Artemisia L. and related genera (Asteraceae, Anthemideae), Plant Biol., 3, 372–378, https://doi.org/10.1055/s-2001-16462, 2001.
Martín, J., Torrell, M., Korobkov, A. A., and Valles, J.: Palynological features as a systematic marker in Artemisia L. and related genera (Asteraceae, Anthemideae) – II: Implications for subtribe Artemisiinae delimitation, Plant Biol., 5, 85–93, https://doi.org/10.1055/s-2001-16462, 2003.
McClelland, H. L. O., Halevy, I., Wolf-Gladrow, D. A., Evans, D., and Bradley, A. S.: Statistical uncertainty in paleoclimate proxy reconstructions, Geophys. Res. Lett., 48, e2021GL092773, https://doi.org/10.1029/2021GL092773, 2021.
Miao, Y. F., Meng, Q. Q., Fang, X. M., Yan, X. L., Wu, F. L., and Song, C. H.: Origin and development of Artemisia (Asteraceae) in Asia and its implications for the uplift history of the Tibetan Plateau: A review, Quatern. Int., 236, 3–12, https://doi.org/10.1016/j.quaint.2010.08.014, 2011.
Mo, R. G., Bai, X. L., Ma, Y. Q., and Cao, R.: On the intraspecific variations of pollen morphology and pollen geography of a relic species-Helianthemum songaricum Schrenk, Acta Bot. Bor-Occid. Sin., 17, 528–532, 1997 (in Chinese).
Moberg, A., Sonechkin, D. M., Holmgren, K., Datsenko, N. M., and Karlen, W.: Highly variable Northern Hemisphere temperatures reconstructed from low- and high-resolution proxy data, Nature, 433, 613–617, https://doi.org/10.1038/nature03265, 2005.
Mosbrugger, V., Utescher, T., and Dilcher, D. L.: Cenozoic continental climatic evolution of Central Europe, P. Natl. Acad. Sci. USA, 102, 14964–14969, https://doi.org/10.1073/pnas.0505267102, 2005.
Olson, D. M., Dinerstein, E., Wikramanayake, E. D., Burgess, N. D., Powell, G. V. N., Underwood, E. C., D'Amico, J. A., Itoua, I., Strand, H. E., Morrison, J. C., Loucks, C. J., Allnutt, T. F., Ricketts, T. H., Kura, Y., Lamoreux, J. F., Wettengel, W. W., Hedao, P., and Kassem, K. R.: Terrestrial ecoregions of the worlds: A new map of life on Earth, Bioscience, 51, 933–938, https://doi.org/10.1641/0006-3568(2001)051[0933:teotwa]2.0.co;2, 2001.
Sánchez-Murillo, R., Durán-Quesada, A. M., Esquivel-Hernández, G., Rojas-Cantillano, D., and Cobb, K. M.: Deciphering key processes controlling rainfall isotopic variability during extreme tropical cyclones, Nat. Commun., 10, 4321, https://doi.org/10.1038/s41467-019-12062-3, 2019.
Sanz, M., Vilatersana, R., Hidalgo, O., Garcia-Jacas, N., Susanna, A., Schneeweiss, G. M., and Vallès, J.: Molecular phylogeny and evolution of floral characters of Artemisia and allies (Anthemideae, Asteraceae): Evidence from nrDNA ETS and ITS sequences, Taxon, 57, 66–78, https://doi.org/10.2307/25065949, 2008.
Shan, B. Q., He, X. L., and Chen, Y. S.: Pollen Morphology of Artemisia in the Loess Plateau, Acta Botanica Boreali-Occidentalia Sinica, 27, 1373–1379, 2007 (in Chinese).
Sing, G. and Joshi, R. D.: Pollen morphology of some Eurasian species of Artemisia, Grana Palynologica, 9, 50–62, https://doi.org/10.1080/00173136909436424, 1969.
Stix, E.: Pollenmorphologische Untersuchungen an Compositen, Grana, 2, 41–104, https://doi.org/10.1080/00173136009429443, 1960.
Sun, J. T. and Xu, Y. T.: Pollen morphology and its taxonomic significance of Artemisia Linn. from Shandong, Journal of Shandong Normal University, 12, 186–190, 1997 (in Chinese).
Sun, X. J., Du, N. Q., Weng, C. Y., Lin, R. F., and Wei, K. Q.: Paleovegetation and paleoenvironment of Manasi Lake, Xinjiang, N. W. China during the last 14 000 years, Quaternary Sciences, 14, 239–248, 1994 (in Chinese).
Sun, X. J., Wang, F. Y., and Song, C. Q.: Pollen-climate response surfaces of selected taxa from northern China, Sci. China Ser. D-Earth Sci., 39, 486–493, 1996.
Tarasov, P. E., Cheddadi, R., Guiot, J., Bottema, S., Peyron, O., Belmonte, J., Ruiz-Sanchez, V., Saadi, F., and Brewer, S.: A method to determine warm and cool steppe biomes from pollen data; application to the Mediterranean and Kazakhstan regions, J. Quaternary Sci., 13, 335–344, https://doi.org/10.1002/(SICI)1099-1417(199807/08)13:4<335::AID-JQS375>3.0.CO;2-A, 1998.
Tierney, J. E., Poulsen, C. J., Montanez, I. P., Bhattacharya, T., Feng, R., Ford, H. L., Honisch, B., Inglis, G. N., Petersen, S. V., Sagoo, N., Tabor, C. R., Thirumalai, K., Zhu, J., Burls, N. J., Foster, G. L., Godderis, Y., Huber, B. T., Ivany, L. C., Turner, S. K., Lunt, D. J., McElwain, J. C., Mills, B. J. W., Otto-Bliesner, B. L., Ridgwell, A., and Zhang, Y. G.: Past climates inform our future, Science, 370, eaay3701, https://doi.org/10.1126/science.aay3701, 2020.
Tutin, T. G., Persson, K., and Gutermann, W.: Artemisia L., in: Flora Europaea Vol. 4, edited by: Tutin, T. G., Heywood, V. H., Burges, N. A., Moore, D. M., Valentine, D. H., Walters, S. M., and Webb, D. A., Cambridge University Press, Cambridge, 178–186, ISBN 978-0521087179, 1976.
Vallès, J., Garcia, S., Hidalgo, O., Martín, J., and Garnatje, T.: Biology, Genome Evolution, Biotechnological issues and research including applied perspectives in Artemisia (Asteraceae), Adv. Bot. Res., 60, 349–419, 2011.
Vrba, E. S.: Evolution, species and fossils-how does life evolve?, S. Afr. J. Sci., 76, 61–84, 1980.
Wang, F. X., Qian, N. F., Zhang, Y. L., and Yang, H. Q.: Pollen morphology of Chinese plants, 2nd edn., Science Press, Beijing, ISBN 7-03-003635-2, 1995 (in Chinese).
Wang, W. M.: On the origin and development of Artemisia (Asteraceae) in the geological past, Bot. J. Linn. Soc., 145, 331–336, https://doi.org/10.1111/j.1095-8339.2004.00287.x, 2004.
Wang, Y., Wang, W., Liu, L. N., Jiang, Y. J., Niu, Z. M., Ma, Y. Z., He, J., and Mensing, S. A.: Reliability of the Artemisia/Chenopodiaceae pollen ratio in differentiating vegetation and reflecting moisture in arid and semi-arid China, Holocene, 30, 858–864, https://doi.org/10.1177/0959683620902219, 2020.
Weng, C. Y., Sun, X. J., and Chen, Y. S.: Numerical characteristics of pollen assemblages of surface samples from the West Kunlun mountains, Acta Bot. Sin., 35, 69–79, 1993 (in Chinese).
Wodehouse, R. P.: Pollen Grain Morphology in the Classification of the Anthemideae, B. Torrey Bot. Club, 53, 479–485, https://doi.org/10.2307/2480028, 1926.
Wu, F. L., Fang, X. M., and Miao, Y. F.: Aridification history of the West Kunlun Mountains since the mid-Pleistocene based on sporopollen and microcharcoal records, Palaeogeogr. Palaeocl., 547, 109680, https://doi.org/10.1016/j.palaeo.2020.109680, 2020.
Wu, Z. Y. (Eds.): Chinese Vegetation, Science Press, Beijing, China, ISBN 7-03-002422-2, 1980 (in Chinese).
Xu, Q. H., Li, Y. C., Yang, X. L., and Zheng, Z. H.: Quantitative relationship between pollen and vegetation in northern China, Sci. China Ser. D-Earth Sci., 50, 582–599, https://doi.org/10.1007/s11430-007-2044-y, 2007.
Yang, J., Spicer, R. A., Spicer, T. E. V., Arens, N. C., Jacques, F. M. B., Su, T., Kennedy, E. M., Herman, A. B., Steart, D. C., Srivastava, G., Mehrotra, R. C., Valdes, P. J., Mehrotra, N. C., Zhou, Z. K., and Lai, J. S.: Leaf form-climate relationships on the global stage: an ensemble of characters, Global Ecol. Biogeogr., 24, 1113–1125, https://doi.org/10.1111/geb.12334, 2015.
Yi, S., Saito, Y., Oshima, H., Zhou, Y. Q., and Wei, H. L.: Holocene environmental history inferred from pollen assemblages in the Huanghe (Yellow River) delta, China: climatic change and human impact, Quaternary Sci. Rev., 22, 609–628, https://doi.org/10.1016/s0277-3791(02)00086-0, 2003a.
Yi, S., Saito, Y., Zhao, Q. H., and Wang, P. X.: Vegetation and climate changes in the Changjiang (Yangtze River) Delta, China, during the past 13 000 years inferred from pollen records, Quaternary Sci. Rev., 22, 1501–1519, https://doi.org/10.1016/s0277-3791(03)00080-5, 2003b.
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K.: Trends, rhythms, and aberrations in global climate 65 Ma to present, Science, 292, 686–693, https://doi.org/10.1126/science.1059412, 2001.
Zachos, J. C., Dickens, G. R., and Zeebe, R. E.: An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics, Nature, 451, 279–283, https://doi.org/10.1038/nature06588, 2008.
Zhang, X. S.: Vegetation map of China and its geographic pattern: Illustration of the vegetation map of the People's Republic of China (1:1 000 000), Geological Press, Beijing, ISBN 978-7-116-04513-2, 2007 (in Chinese).
Zhang, Y., Kong, Z. C., Wang, G. H., and Ni, J.: Anthropogenic and climatic impacts on surface pollen assemblages along a precipitation gradient in north-eastern China, Global Ecol. Biogeogr., 19, 621–631, https://doi.org/10.1111/j.1466-8238.2010.00534.x, 2010.
Zhang, Y. N. and Qian, C.: SEM observation on pollen morphology of lily species, Acta Pratac. Sin., 20, 111–118, 2011 (in Chinese).
Zhao, X. L. and Yao, C. H.: Pollen Morphlogy differences among Osmanthus fragrans cultivars, J. Hubei Minzu Univ. (Nat. Sci. Ed.), 17, 16–20, 1999 (in Chinese).
Zhao, Y., Xu, Q. H., Huang, X. Z., Guo, X. L., and Tao, S. C.: Differences of modern pollen assemblages from lake sediments and surface soils in arid and semi-arid China and their significance for pollen-based quantitative climate reconstruction, Rev. Palaeobot. Palyno., 156, 519–524, https://doi.org/10.1016/j.revpalbo.2009.05.001, 2009.
Zhao, Y., Liu, H. Y., Li, F. R., Huang, X. Z., Sun, J. H., Zhao, W. W., Herzschuh, U., and Tang, Y.: Application and limitations of the ArtemisiaChenopodiaceae pollen ratio in arid and semi-arid China, Holocene, 22, 1385–1392, https://doi.org/10.1177/0959683612449762, 2012.
Zhao, Y. T., Miao, Y. F., Fang, Y. M., Li, Y., Lei, Y., Chen, X. M., Dong, W. M., and An, C. B.: Investigation of factors affecting surface pollen assemblages in the Balikun Basin, central Asia: Implications for palaeoenvironmental reconstructions, Ecol. Indic., 123, 107332, https://doi.org/10.1016/j.ecolind.2020.107332, 2021.
Zizka, A., Silvestro, D., Andermann, T., Azevedo, J., Ritter, C. D., Edler, D., Farooq, H., Herdean, A., Ariza, M., Scharn, R., Svantesson, S., Wengstrom, N., Zizka, V., and Antonelli, A.: CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases, Methods Ecol. Evol., 10, 744–751, https://doi.org/10.1111/2041-210X.13152, 2019.