the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Patos Lagoon estuary and adjacent marine coastal biodiversity long-term data
Valéria M. Lemos
Marianna Lanari
Margareth Copertino
Eduardo R. Secchi
Paulo Cesar O. V. de Abreu
José H. Muelbert
Alexandre M. Garcia
Felipe C. Dumont
Erik Muxagata
João P. Vieira
André Colling
Clarisse Odebrecht
Estuaries are among the most productive aquatic ecosystems and provide important ecological and economic services in coastal areas. However, estuarine systems have been threatened worldwide by natural and anthropogenic impacts acting on local, regional, and global scales. Long-term ecological studies contribute to the understanding and management of estuarine functioning and provide the baseline information for detection changes and modeling of predictive scenarios. Here, we describe long-term data on the biodiversity and physico-chemical parameters obtained from 1993 to 2016 for the Patos Lagoon estuary and adjacent marine coast (PLEA), in southern Brazil. We report 8 datasets containing 6972 sampling events with the occurrence and abundance records of 275 species (kingdoms: Bacteria, Protozoa, Chromista, Plantae, and Animalia) of functional groups plankton, benthos, and nekton. Datasets also include 22 190 abiotic records. The database is published in the Global Biodiversity Information Facility (GBIF) repository (see Sect. 3 “Data availability” and Table 3). The present compendium represents one of the most comprehensive and longest datasets from primary producers to top predators in an estuarine coastal system in South America, and their availability will be an important contribution to the understanding and predictability of estuarine dynamics around the world.
- Article
(2198 KB) - Full-text XML
- BibTeX
- EndNote
Coastal and marine biodiversity are facing an unprecedented worldwide threat from climate change, pollution, overfishing, habitat destruction, and invasive species (Lotze et al., 2006; Christian and Mazzilli, 2007; Halpern et al., 2008; Kennish and Paerl, 2010; Doney and Schimel, 2015), impairing the ecosystem functions and the delivery of goods and services to society. The comprehension of most of those threats requires knowledge of the long-term variability in both biological and environmental variables, which are the baseline for ecological studies and for the detection of early warning signals of natural and anthropogenic impacts and the modeling of predictive scenarios (Vihervaara et at., 2013; Muelbert et al., 2019). The establishment and maintenance of ecological observations of coastal ecosystems are crucial to support scientists and stakeholders with the necessary information to quantify environmental changes and their impact on the biodiversity and the sustainable use of the seas and coasts (Muelbert et al., 2019). That information is crucial to implement conservation and sustainable development targets (e.g., evaluating progress toward Aichi targets of the Convention on Biological Diversity (CBD; Dreujou et al., 2020) and several of the UN Sustainable Development Goals) and to enable global assessments such as those by the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES) and the UN World Ocean Assessment (Duffy et al., 2013).
Our current understanding of marine ecosystem responses to human activities is limited by the availability of data, particularly long-term series of physical, chemical, and biological conditions (Carstensen, 2014). Despite the important global initiatives (e.g., International Long-Term Ecological Research, ILTER), long-term, integrated, ecosystem-level monitoring efforts are still scarce for most coastal and marine ecosystems (Kennish and Paerl, 2010; Duffy et al., 2013; Vihervaara et al., 2013; Muelbert et al., 2019), particularly in the Southern Hemisphere (Odebrecht et al., 2017). The scarcity of time series from coastal ecosystems hampers the assessment of the impacts and undermines our ability to respond effectively to these threats (Turra and Denadai, 2016).
Estuaries and nearshore coastal regions are some of the most productive ecosystems on earth (McLusky and Elliott, 2004), yet they are among the most affected by human activities and climate change (Ruiz et al., 2000). In southern Brazil, the Patos Lagoon estuary and adjacent marine coast (PLEA) have been long recognized (Von Ihering, 1885) by their high biological productivity, together with human interference (Odebrecht et al., 2017). Considered the largest choked lagoon in the world (Kjerfve, 1986), the Patos Lagoon connects the continental waters to the western South Atlantic Ocean and performs a critical role in the regional economy (Seeliger, 2001). The favorable natural conditions and strategic position led to the development of local and regional economic activities associated with artisanal and industrial fisheries (Kalikoski and Vasconcellos, 2012; Haimovici et al., 2014; Haimovici and Cardoso, 2017), port activities, industry, and tourism (Newton et al., 2018).
The PLEA has been a site of the Brazilian Long-Term Ecological Research (LTER) program since 1998, although oceanographic and ecological studies started in the 1970s (Odebrecht et al., 2017). The LTER-PLEA (PELD-ELPA in Portuguese) is a well-established and consolidated monitoring program, producing some of the longest datasets on estuarine and marine biota and abiotic parameters in the Southern Hemisphere (Odebrecht et al., 2010, 2017). Together with other ILTER coastal and marine sites (about 120 around the globe) (Muelbert et al., 2019), the LTER-PLEA has great potential to contribute to global coastal and ocean observation.
The LTER-PLEA contributes information about the biota composition, distribution, and abundance at seasonal, interannual, and decadal timescales, providing the basis to understand the estuarine ecological processes and their driving forces. Many studies have demonstrated how the variability in climate and hydrology influences the ecology of estuarine and marine biodiversity and production (Seeliger and Odebrecht, 2010; Odebrecht et al., 2010, 2017). PLEA is affected by large-scale and remote phenomena, the most important being the El Niño–Southern Oscillation (ENSO), which strongly influences southern South America precipitation and fluvial discharge (Robertson and Mechoso, 1997; Grimm et al., 1998). The interactions among climate and hydrology directly affect the dynamics of plankton (Muxagata et al., 2012; Haraguchi et al., 2015; Odebrecht et al., 2015; Abreu et al., 2016; Teixeira-Amaral et al., 2017; Salvador and Muelbert, 2019), macroalgae, seagrasses, benthic invertebrates (Colling et al., 2010; Lanari and Copertino, 2017; Lanari et al., 2018), and fish (Garcia et al., 2001, 2003; Vieira et al., 2010; Moraes et al., 2012; Garcia et al., 2017), with implications for species conservation (Costa et al., 2016), including fishing resources (Castello and Möller, 1978; Odebrech and Castello, 2001; Vieira et al., 2008). Impacts of human activities such as overfishing and dredging, combined with the ENSO, have the potential to affect biodiversity and ecological functions. Therefore, the PLEA is an ideal environment to test hypotheses about changes in biodiversity and their functioning at several temporal scales.
Here, we describe a database comprising biological parameters within plankton, benthos, and nekton communities, from primary producers to top predators, and associated water physical–chemical parameters. The compendium represents one of the most robust and longest databases of biological diversity in an estuarine coastal system of South America. The dataset is the framework for understanding the structure and functioning of PLEA and can be an important tool for environmental management and decision-making. Furthermore, the data provide information for the modeling of predictive scenarios of climate change impacts, which are fundamental for local adaptation and mitigation strategies, but also for a better understanding of coastal environment dynamics and functioning.
2.1 Geographical coverage
The Patos Lagoon estuary is part of the Patos–Mirim lagoon system located in the subtropical coastal plain of southern Brazil (Fig. 1). The geographical coverage of the dataset ranges from to S latitude and from to W longitude. The estuarine region (∼10 360 km2) consists mainly of shallow areas (i.e., 75 % of the total area is <2 m), except for natural channels (3–5 m deep) and the main navigation channel (∼14 m deep). The estuary receives freshwater from a 200 000 km2 drainage basin and is under the influence of a microtidal regime (∼0.47 m) strongly attenuated by the single and narrow entrance channel (0.5 to 3 km wide). Hydrology is driven mainly by fluvial discharge and wind patterns, and on an annual basis, the estuary can be river-dominated, display a salt wedge, and become a partially mixed or even a well-mixed system (Möller et al., 2001). Marine and euhaline conditions occur in summer and autumn, whereas oligohaline conditions in general prevail in winter and spring (Möller et al., 2001).
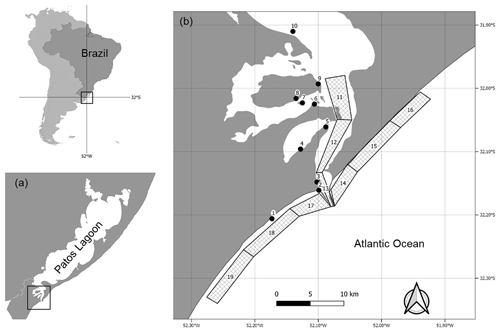
Figure 1Study area. (a) Patos Lagoon, southern Brazil. (b) Map of the sampling points in the Patos Lagoon estuary and adjacent marine coast (PLEA) for the LTER-PLEA's dataset samplings. The sampling points are described in Table 1.
Interannual variability in hydrological patterns is largely associated with ENSO remote effects on regional precipitation, with anomalous high and low freshwater run-off occurring during El Niño and La Niña years, respectively (Odebrecht et al., 2010). Overall, high levels of nutrients in the water column (up to 40 µM , 40 µM , and 8.7 µM ) and sediment (up to 710.7 µM and 14.6 µM ) are maintained through inputs from the watershed, macrophytes, and anthropogenic sources (Baumgarten and Niencheski, 2010; Odebrecht et al., 2010).
2.2 Data description
The data described are a product of the Brazilian Long-Term Ecological Research program in the Patos Lagoon estuary and adjacent marine coast – LTER-PLEA, established in 1998. The program aims to investigate the main natural and anthropic impacts on biotic and abiotic components of this ecosystem. Distinct areas of the PLEA have been monitored, generating a core set of measurements repeated over time across spatial gradients (Fig. 1 and Table 1). Eight datasets, covering the biota (phyto-, zoo-, and ichthyoplankton; benthic macrofauna; seagrasses; macroalgae; pink shrimp; fish and marine mammals such as dolphins) and associated physical and chemical water parameters (salinity, temperature, transparency, chlorophyll a, inorganic dissolved nutrients, and seston), were obtained by several research groups and laboratories that systematically and almost simultaneously monitor the PLEA (Fig. 2a) at different temporal scales (daily, monthly, and seasonally) (Table 2). Some datasets include sampling since 1993, prior to the start of the LTER-PLEA (Fig. 2a).
Table 1Geographic locations at sampling stations of the Brazilian Long-Term Ecological Research program in the Patos Lagoon estuary and adjacent marine coast, LTER-PLEA.
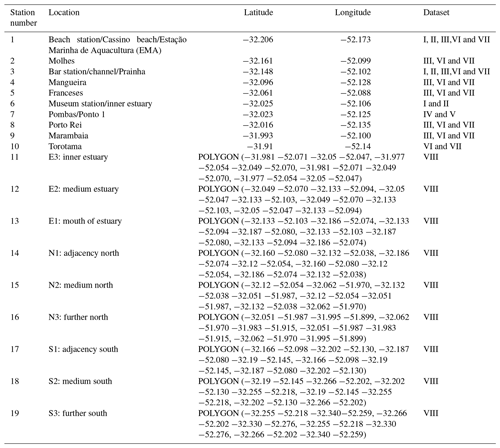
Dataset I: phytoplankton and water quality parameters in the Patos Lagoon estuary and adjacent marine coast; dataset II: continuous monitoring of the micro- and mesozooplankton of the Patos Lagoon estuary and adjacent coastal area; dataset III: interannual variability in ichthyoplankton diversity in the Patos Lagoon estuary in southern Brazil; dataset IV: dynamics of submerged aquatic vegetation in the Patos Lagoon estuary; dataset V: temporal data series of benthic macrofauna abundance and composition from the Patos Lagoon estuary; dataset VI: ecology of the pink shrimp Penaeus paulensis in Patos Lagoon estuary; dataset VII: species composition and abundance patterns of fish assemblages in shallow waters of Patos Lagoon estuary; dataset VIII: ecology of Lahille's bottlenose dolphin Tursiops truncatus gephyreus in the Patos Lagoon estuary and adjacent marine coast. WGS84 geodetic datum.
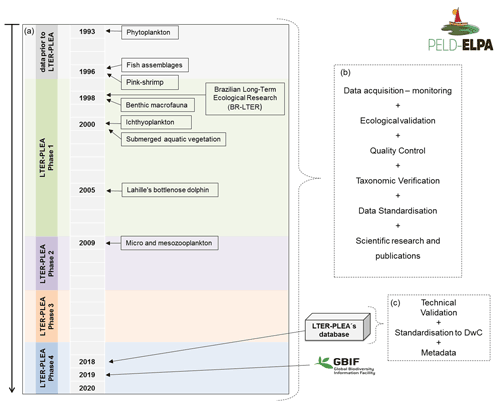
Figure 2Stages of the LTER-PLEA's database life cycle: from data collection to integration in the Global Biodiversity Information Facility (GBIF). (a) LTER-PLEA timeline; (b) quality assurance and control process; (c) technical validation process, formatting, and registration of the LTER-PLEA's database in the GBIF.
Table 2Temporal coverage of the LTER-PLEA's datasets.
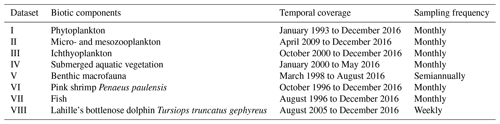
Dataset I: phytoplankton and water quality parameters in the Patos Lagoon estuary and adjacent marine coast; dataset II: continuous monitoring of the micro- and mesozooplankton of the Patos Lagoon estuary and adjacent coastal area; dataset III: interannual variability in ichthyoplankton diversity in the Patos Lagoon estuary in southern Brazil; dataset IV: dynamics of submerged aquatic vegetation in the Patos Lagoon estuary; dataset V: temporal data series of benthic macrofauna abundance and composition from the Patos Lagoon estuary; dataset VI: ecology of the pink shrimp Penaeus paulensis in Patos Lagoon estuary; dataset VII: species composition and abundance patterns of fish assemblages in shallow waters of Patos Lagoon estuary; dataset VIII: ecology of Lahille's bottlenose dolphin Tursiops truncatus gephyreus in the Patos Lagoon estuary and adjacent marine coast.
2.3 Data sources and sampling protocol
The eight datasets were based on the use of distinct sampling strategies and methods, according to the goals and specific characteristics of the biotic and abiotic components investigated. Consistency in data collection was continually emphasized and was assisted by continued participation of the same researchers over the time period.
2.3.1 Phytoplankton (dataset I)
Sampling description. Phytoplankton was sampled monthly at three stations located in the PLEA (Fig. 1 and Table 1). Phytoplankton for qualitative analysis was sampled by horizontal tows using a 20 µm mesh size plankton net and stored in glass bottles fixed with formaldehyde (4 %) neutralized with hexamethylenetetramine. For phytoplankton quantitative (cells counting) analysis, surface water samples were stored in amber glass bottles and fixed with lugol's solution (2 %). Phytoplankton composition and abundance were obtained using the classical Utermöhl sedimentation method and described elsewhere (Odebrecht et al., 2010; Haraguchi et al., 2015). Six persons (graduate students, technician, researcher) counted phytoplankton over the whole study period using the same procedure, i.e., screening of half (density of predominant species >100 units) or the entire sedimentation chamber for organisms larger than 50 µm under low magnification (100×) using an optical microscope (inverted microscope, Zeiss). Smaller organisms were counted according to the cell density, under magnification of 200× and/or 400×, in strips of at least 30 fields.
The routine identification of phytoplankton morpho-species was conducted under optical microscopy (inverted and transmitted light, Zeiss) and followed the classical literature (Balech, 1988; Tomas, 1996, 1997; Hoppenrath et al., 2009) and specific taxonomic articles. Electron microscopy was used for the identification of some species, e.g., Skeletonema (Bergesch et al., 2009) and Pseudo-nitzschia (Hagström et al., 2011). Thalassiosira, however, was identified and counted at the genus level due to identification difficulties using the optical microscope (Garcia and Odebrecht, 2009). Other difficulties identifying species were grouped at higher taxonomic levels (i.e., centric and pennate diatoms, armored or unarmored gymnodinioid dinoflagellates). Also, small flagellates (<20 µm) and coccoid cells were grouped and counted in size classes. Molecular biology tools were applied to species of Asterionellopsis (Franco et al., 2016) and Pseudo-nitzschia (Hagström et al., 2011). Non-identifiable or damaged specimens associated with the phytoplankton sample were annotated as NA (not available). The autotrophic ciliate Mesodinium was included in the present dataset.
The physical water parameters were obtained in situ: temperature (mercury thermometer), salinity (optical refractometer or conductivity meter, YSI model 33 SCT – salinity, conductivity, temperature), and water transparency (Secchi disk). Surface water samples were obtained using a bucket, stored in plastic bottles and maintained in the dark for the analysis of dissolved inorganic nutrients and chlorophyll a (Abreu et al., 2010, 2016). In the laboratory, 50–100 mL water aliquots were filtered (Whatman GF/F glass fiber filters) and frozen (−40 ∘C) for the analysis of dissolved inorganic silicate, phosphate, and nitrite + nitrate, while ammonium and chlorophyll a (material retained on the filters) analysis was conducted right away. The concentration of the former nutrients was measured according to the methods described by Strickland and Parsons (1972), and that of ammonium followed the method of UNESCO (1983). For the chlorophyll a concentration analysis, pigments were extracted (acetone solution 90 % ) in the dark and cold for 24 h and measured using a calibrated Turner Designs fluorometer with correction for degradation products (Strickland and Parsons, 1972; Welschmeyer, 1994).
Quality assurance (QA) and quality control (QC). The same researchers and methods were employed since the beginning of the study period. Thus, methodology influence is minimal. People involved in sampling activities and technical assistance for nutrient analysis and phytoplankton counting changed during the years (six persons), but always under supervision of the same responsible researchers. However, each change in personnel responsible for the determination of cell abundance occurred after training and under the supervision of a senior researcher in this area. When technical assistance changed, duplicate analyses were carried out for quality control, which guaranteed the high quality of the data throughout the project. The species nomenclature was updated following the evolution of new taxonomic and systematic studies.
2.3.2 Micro- and mesozooplankton (dataset II)
Sampling description. Zooplankton samples were collected monthly at three stations located in the PLEA (Fig. 1 and Table 1). Zooplankton samples at the estuary were collected from sub-superficial horizontal tows of ∼3 min using mini bongo frames 30 cm in diameter fitted with 200 and 90 µm meshes with calibrated Hydro-Bios flowmeters attached to the mouth of each net. For the adjacent marine coast (Cassino Beach), we used a conventional 30 cm diameter frame fitted with a 200 µm mesh and a calibrated TSK flowmeter. All samples were immediately preserved in a 4 % borax-buffered formaldehyde solution (Steedman, 1976) until processing.
All zooplankton organisms present in subsamples of 1.25 % to 50 % of the 200 µm mesh samples were counted on a stereoscopic microscope and identified at the lowest taxonomic level possible. All results are expressed in numbers of organisms per cubic meter. Copepod species were identified according to Rose (1933), Björnberg (1981), and Bradford-Grieve (1999); barnacle nauplii according to Lang (1979, 1980); and the remaining species and/or groups using specific references from Boltovskoy (1981, 1999). Non-identifiable or damaged specimens were recorded as NA (not available).
At each station water samples of 20 to 100 mL were collected and filtered through 2.5 cm glass microfiber filters (Whatman GF/F) using a syringe with a filter holder attachment (Swinnex). Each filter paper was then folded in half, wrapped in foil, and frozen until processing. Chlorophyll a was extracted with 90 % acetone, and readings were made on a calibrated Turner Designs fluorometer (TD 700) according to Welschmeyer (1994). Temperature and salinity were also measured at each station using a thermosalinometer (Hanna HI 9828), refractometers, and a mercury thermometer, depending on availability.
Quality assurance (QA) and quality control (QC). All samples were collected under the supervision of planktologists or graduate students of the zooplankton laboratory, and the taxonomic quality of the data was checked before uploading to the database.
2.3.3 Ichthyoplankton (dataset III)
Sampling description. Ichthyoplankton samples were collected monthly at seven stations located in the PLEA (Fig. 1 and Table 1). Sampling of fish eggs and larvae was made with a 50 cm diameter 300 µm mesh conical net equipped with a flowmeter. The net was manually hauled to the beach area by two people over a duration of 2 min at the seven stations. In the laboratory, samples were concentrated in 300 mL jars, and all fish eggs and larvae were sorted from the remaining zooplankton. All the collected material was preserved in 4 % formaldehyde.
In the laboratory, the material was screened and identified. Fish eggs and larvae were identified at the lowest taxonomic level possible according to Weiss (1981), Fahay (1983), Moser (1996), Olivar et al. (1999), and Richards (2005). Non-identifiable or damaged specimens were recorded as NA (not available). Total abundance was recorded by taxa as an absolute number and standardized to a volume of 100 m3.
Temperature and salinity were registered with a YSI thermosalinometer with precision of 0.01 ∘C and of 0.01 salinity units.
Quality assurance (QA) and quality control (QC). The sampling and laboratory procedure were conducted under the same supervision and with the same standardized technique during this period. Taxonomic determination was done by the same qualified technical expert for the entire period so far. Data were double-checked for typing errors and inadequate values.
2.3.4 Submerged aquatic vegetation (dataset IV)
Sampling description. The submerged aquatic vegetation (SAV) was monitored monthly in a shallow area historically occupied by Ruppia maritima meadows and drift green macroalgae at an inner and protected shoal within the mesomixohaline region of Patos Lagoon estuary (Fig. 1 and Table 1). The sampling design aimed to cover the center of the meadow and their ending limits, covering approximately 1500 m2. The meadow was surveyed across a 500 m fixed transect parallel to the coast and 500 m from Pombas Island. The permanent transect was marked by six fixed wooden posts, buried 2–3 m into the sediment. Surrounding each fixed post, the percentage cover of rooted plants and macroalgae was quantified within randomized 25×25 cm quadrats (N=3). Within each quadrat, the plant biomass was sampled by using cylindrical cores, buried into the sediment (ø 10 cm, 15 cm depth) (N=3 per plot, total N=18). The samples were previously washed in the field with the help of sieves and were packed and transported to the laboratory with ice.
In the laboratory, the biomass samples were washed and cleaned from sediments, debris, and associated fauna. The plant biomass was split into functional groups: rooted plants, macroalgae, and epiphyte algae. The rooted plants were divided into belowground and aboveground fractions. Plant development and phenology were recorded. Epiphyte algae were removed by scraping the leaves with a surgical blade. Demographic parameters were obtained for the rooted plants such as hast length, leaf length, number of shoots, total rhizome length, and number of nodes along the rhizome. The dry weight (48 h at 60 ∘C) was obtained for each functional group and rooted plant compartments (belowground and aboveground).
Quality assurance (QA) and quality control (QC). Samples were always collected by researchers or trained students. Soon after sample trial and parameter measurements, data were compiled, and basic statistics (average, standard deviations) were calculated. Data were visualized and analyzed by graphical exploration approaches for detecting anomalies and errors. In addition, new data were always checked against historical data for comparison and identification of patterns.
Technical information. With the drastic reduction in seagrass abundance detected in Patos Lagoon and across the Brazilian coast (Copertino et al., 2016), the methodology was re-evaluated to integrate a national network approach. Therefore, this particular SAV dataset was discontinued in 2016, and a new field protocol was initiated according to the benthic monitoring network (known as the ReBentos protocol in Brazil) (Copertino et al., 2015). The ReBentos protocol includes surveys and observations at three sites within the Patos Lagoon estuary, exposed to different environmental conditions (salinity, transparency, nutrient input, anthropogenic impacts). In addition to percentage cover, biomass, and demographic parameters recorded across three transects (at each site), the ReBentos protocol includes information about sediment and water parameters and seagrass meadow area.
2.3.5 Benthic macrofauna (dataset V)
Sampling description. The benthic macrofauna samples were collected at four stations located in the estuary of the Patos Lagoon (Fig. 1 and Table 1). Data have been acquired twice a year, comprising winter (August) and summer (March) samplings. The samples were taken with a 10 cm diameter PVC corer (0.0078 m2) buried 20 cm into the substrate and sieved through a 300 µm mesh (Gray and Elliott, 2009). All collected material was fixed in 4 % formaldehyde and preserved in ethanol (70 %).
The macrofauna specimens were identified at the lowest taxonomic level (Amaral and Nonato, 1996; Buckup and Bond-Buckup, 1999; Rios, 2009) and quantified with the aid of stereomicroscopes (40×) in the laboratory. The abundance data for each point represent the number of organisms for each species collected by the PVC corer.
Quality assurance (QA) and quality control (QC). Sampling was done by qualified technical experts continuously trained to use the same techniques and methods. The quality of data was checked monthly before the uploading to the database. Data were checked for typing errors under supervision of experienced researchers.
2.3.6 Pink shrimp Penaeus paulensis (dataset VI)
Sampling description. The pink shrimp samples were collected monthly at eight stations (Fig. 1 and Table 1) located in the PLEA with five replicates each. The gear used to obtain biological samples was a beach seine (9 m in length, 13 mm (adjacent knots) and 5 mm in the center), an active net designed to operate in shallow regions (average depth lower than 1.5 m). The pink shrimp samples were preserved in 10 % formalin and later identified in the laboratory according to Buckup and Bond-Buckup (1999).
Water temperature (mercury thermometer), salinity (refractometer), and transparency (Secchi disk) data were collected at each sampling occasion.
Quality assurance (QA) and quality control (QC). Researchers and the classical methods were the same since the beginning of the time series. Thus, the methodology influence is minimal. Sampling was always performed under supervision of experienced researchers.
2.3.7 Fish assemblages (dataset VII)
Sampling description. The ichthyofauna samples were collected monthly at eight stations located in the PLEA (Fig. 1 and Table 1). Fish were sampled using a 9 m beach seine (13 mm bar mesh in the wings and 0.5 mm mesh adjacent knots in the 3 m center section) that was pulled to cover an area of about 60 m2 during each haul. Five hauls were made monthly (usually in the first week of each month) at each sampling station. After sampling, fish were euthanized using eugenol (CONCEA, 2013) and stored in plastic bags with 10 % formalin. In the laboratory, fish were transferred to 70 % alcohol. Fish were then identified at the lowest taxonomic level possible according to the specialized literature, such as Figueiredo (1977), Menezes and Figueiredo (1980, 1985), Figueiredo and Menezes (1978, 1980, 2000), Fischer et al. (2004), and FishBase (Froese and Pauly, 2020).
Concomitant with fish sampling, the water temperature (mercury thermometer), salinity (refractometer), and transparency (Secchi disk) data were collected at each station. Datasets VI and VII are subsets of the same sampling scheme, and the associated environmental variables are exactly the same.
Quality assurance (QA) and quality control (QC). Principal investigators and fishing gear were the same since the beginning of the time series. Fish sampling at one of the sampling stations (Marambaia) had to be discontinued in 2013 due to physical changes at the site that prevented beach seining. Sampling and species identification methods were consistent across the years of the study and occurred under the supervision of the same principal investigators.
2.3.8 Lahille's bottlenose dolphin Tursiops truncatus gephyreus (dataset VIII)
Sampling description. The area sampled is 140 km2 and encompasses the lower portion of the Patos Lagoon estuary (40 km2) and adjacent marine coast (50 km2 south and 50 km2 north of Patos Lagoon estuary in the coastal zone) (Fig. 1 and Table 1).
Distribution patterns and habitat use. All fieldwork was carried out onboard a 5.6 m long inflatable boat equipped with a 90 hp outboard engine, VHF radio, echo sounder, and GPS. Boat-based surveys were conducted year-round in the core area of the Patos Lagoon estuary dolphin community. Surveys in estuarine waters were conducted following predefined zigzag transects, whereas in the adjacent marine coast either zigzag or parallel-to-shore transects up to 20 km north and south of the estuary mouth were run, depending on the sea conditions and objective of the sampling occasion. This variation, however, does not interfere with the nature and quality of data inserted in the database. Surveys were halted when the sea state (Beaufort scale) was >3. Transects were run at speeds between 18 and 22 km h−1. Two observers positioned in the bow searched for dolphins visually. Whenever a dolphin group was sighted, the survey route was abandoned to approach the animals for photo-identification; for skin or blubber biopsy sampling; and to obtain data of group size and composition, sighting depth, and geographical position. After a sufficient number of good-quality digital photographs of the dorsal fins of presumably all animals were taken, the survey was resumed.
Quality assurance (QA) and quality control (QC). Principal investigators and major sampling protocols remained the same since the beginning of the study. Adaptations to sampling procedures were eventually made for specific research questions but do not interfere with the content and quality of the dataset presented in this compendium.
2.4 Data management and standardization
The LTER-PLEA's database sharing was performed based on the FAIR principles, which ensures that all data are easily findable, accessible, interoperable, and reusable (Wilkinson et al., 2016; Tanhua et al., 2019). Thus, the potential of these data is summarized as “findable” by the integration and dissemination of data and metadata through the Global Biodiversity Information Facility (GBIF; https://www.gbif.org, last access: 9 October 2021) with unique and persistent identifiers assigned (Table 3), “accessible” by open and free access to the data and metadata through friendly tools, “interoperable” by standardizing data in an internationally widely accepted controlled vocabulary and spreadsheet structure, and “reusable” by providing a complete description of the dataset in GBIF metadata sections and in the presented data descriptor. Data management followed the steps below (Fig. 2b and c):
- 1.
Data holders supplied their datasets to the database manager in digital format (e.g., spreadsheets, csv files).
- 2.
Data were checked by the researchers responsible for data and by the data manager for automatic and manual corrections (see quality assurance and quality control in Sect. 2.3 for more details).
- 3.
Datasets were formatted according to the Darwin Core (DwC) standard (TDWG, 2015) and the OBIS-ENV-DATA format (De Pooter et al., 2017).
- 4.
Technical validation was performed (taxonomic and structural validation).
- 5.
The metadata were described and verified by the responsible researchers.
- 6.
The resulting database was published in the GBIF platform through the Integrated Publishing Toolkit (IPT) provided by the Information System on Brazilian Biodiversity (SiBBr; https://www.sibbr.gov.br, last access: 20 July 2021), which is the Brazilian node of the GBIF.
2.5 Dataset structure
All datasets are available as a Darwin Core Archive (DwC-A), and all fields were named compliant with Darwin Core (DwC) standards (TDWG, 2015). The DwC offers a stable and flexible framework to store all fields available in original data sources. Each dataset is published as sampling event data, formatted using a star scheme based on the OBIS-ENV-DATA format (De Pooter et al., 2017), which includes an event core (event sampling data), occurrence (taxonomic data), and extended measurement or fact (environmental variables and taxa abundances) (Fig. 3).
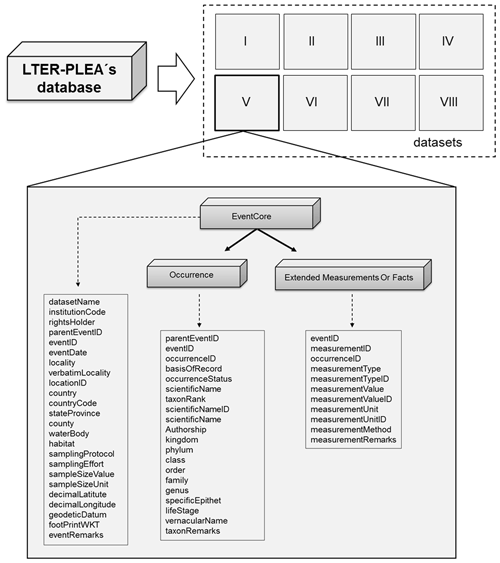
Figure 3The LTER-PLEA's dataset structure. The LTER-PLEA's dataset structure based on the OBIS-ENV-DATA format. Darwin Core terms (https://dwc.tdwg.org/terms/, last access: 10 October 2020) used in each extension are described in the boxes. Dataset I: phytoplankton and water quality parameters in the Patos Lagoon estuary and adjacent marine coast; dataset II: continuous monitoring of the micro- and mesozooplankton of the Patos Lagoon estuary and adjacent coastal area; dataset III: interannual variability in ichthyoplankton diversity in the Patos Lagoon estuary in southern Brazil; dataset IV: dynamics of submerged aquatic vegetation in the Patos Lagoon estuary; dataset V: temporal data series of benthic macrofauna abundance and composition from the Patos Lagoon estuary; dataset VI: ecology of the pink shrimp Penaeus paulensis in Patos Lagoon estuary; dataset VII: species composition and abundance patterns of fish assemblages in shallow waters of Patos Lagoon estuary; dataset VIII: ecology of Lahille's bottlenose dolphin Tursiops truncatus gephyreus in the Patos Lagoon estuary and adjacent marine coast.
Each sampling event formed one row in the event core data table and was identified by an event ID code that is a unique identifier of each sampling event (something that occurs at a place and time) (TDWG, 2015) and that was built according to the following information: (1) the standard code for the country (Brazil: Br), (2) project name (i.e., the source organization; in Portuguese: PELD-ELPA), (3) the identifier for the institution with custody of the information referred to in the record (Universidade Federal de Rio Grande, FURG), (4) locality name (Patos Lagoon), (5) dataset name, (6) year, (7) month, and (8) identifier of the sampling station.
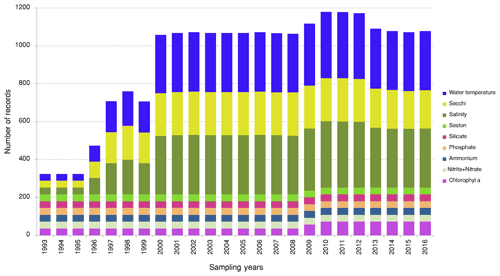
The LTER-PLEA's database contains a total of 6972 sampling event records unequally distributed across the research groups during the monitoring period (Fig. 4). The sampling events encompassed 106 155 taxa occurrences and 22 190 abiotic measurements (Fig. 5) in the Patos Lagoon estuary and adjacent marine coast.
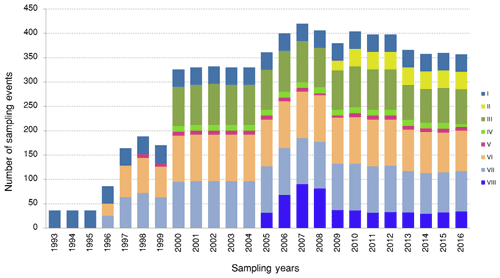
Figure 4Number of sampling events for datasets (biota monitoring) in the Patos Lagoon estuary and adjacent marine coast from 1993 to 2016. The plot shows the number of sampling events collected for each year and for each dataset. Dataset I: phytoplankton and water quality parameters in the Patos Lagoon estuary and adjacent marine coast; dataset II: continuous monitoring of the micro- and mesozooplankton of the Patos Lagoon estuary and adjacent coastal area; dataset III: interannual variability in ichthyoplankton diversity in the Patos Lagoon estuary in southern Brazil; dataset IV: dynamics of submerged aquatic vegetation in the Patos Lagoon estuary; dataset V: temporal data series of benthic macrofauna abundance and composition from the Patos Lagoon estuary; dataset VI: ecology of the pink shrimp Penaeus paulensis in Patos Lagoon estuary; dataset VII: species composition and abundance patterns of fish assemblages in shallow waters of Patos Lagoon estuary; dataset VIII: ecology of Lahille's bottlenose dolphin Tursiops truncatus gephyreus in the Patos Lagoon estuary and adjacent marine coast.
2.6 Taxonomic coverage
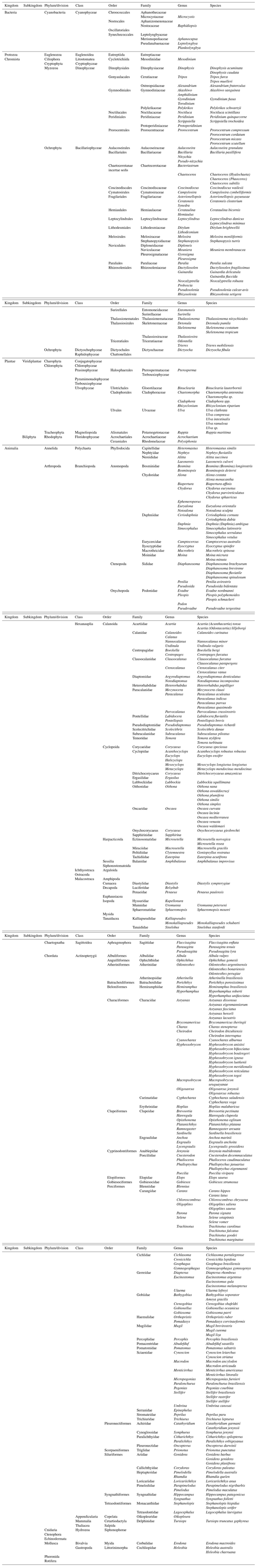
General taxonomic coverage description. The 8 datasets comprise the description of the occurrence of 21 phyla, 30 classes, 78 orders, 142 families, 232 genera, and about 275 species from the 5 kingdoms (Bacteria, Protozoa, Chromista, Plantae, and Animalia; Appendix A).
2.7 Dataset taxonomic coverage
2.7.1 Phytoplankton (dataset I)
Taxonomic ranks.
Kingdoms: Bacteria, Chromista, Plantae, and Protozoa.
Phyla: Charophyta, Chlorophyta, Ciliophora, Cryptophyta, Cyanobacteria, Euglenozoa, Myzozoa, and Ochrophyta.
Class: Bacillariophyceae, Chlorophyceae, Conjugatophyceae, Cryptophyceae, Cyanophyceae, Dictyochophyceae, Dinophyceae, Euglenoidea, Litostomatea, Prasinophyceae, Pyramimonadophyceae, Raphidophyceae, Trebouxiophyceae, and Ulvophyceae.
Orders: Aulacoseirales, Bacillariales, Chaetocerotanae incertae sedis, Chattonellales, Chroococcales, Coscinodiscales, Cyclotrichiida, Cymatosirales, Dictyochales, Dinophysiales, Eutreptiida, Fragilariales, Gonyaulacales, Gymnodiniales, Halosphaerales, Hemiaulales, Leptocylindrales, Lithodesmiales, Melosirales, Naviculales, Noctilucales, Nostocales, Oscillatoriales, Paraliales, Peridiniales, Prorocentrales, Rhizosoleniales, Surirellales, Synechococcales, Thalassionematales, Thalassiosirales, Triceratiales, and Ulotrichales.
Families: Aphanizomenonaceae, Aphanothecaceae, Aulacoseiraceae, Bacillariaceae, Ceratiaceae, Chaetocerotaceae, Coscinodiscaceae, Cymatosiraceae, Dictyochaceae, Dinophysiaceae, Diploneidaceae, Entomoneidaceae, Eutreptiaceae, Fragilariaceae, Gloeotilaceae, Gonyaulacaceae, Gymnodiniaceae, Hemiaulaceae, Leptocylindraceae, Leptolyngbyaceae, Lithodesmiaceae, Melosiraceae, Merismopediaceae, Mesodiniidae, Microcystaceae, Naviculaceae, Noctilucaceae, Nostocaceae, Paraliaceae, Peridiniaceae, Pleurosigmataceae, Polykrikaceae, Prorocentraceae, Protoperidiniaceae, Pseudanabaenaceae, Pterospermataceae, Rhizosoleniaceae, Skeletonemaceae, Stephanopyxidaceae, Surirellaceae, Thalassionemataceae, Thalassiosiraceae, and Triceratiaceae.
Genera: Akashiwo, Alexandrium, Amphidinium, Aphanocapsa, Asterionellopsis, Aulacoseira, Bacillaria, Bacteriastrum, Binuclearia, Campylosira, Cerataulina, Ceratoneis, Chaetoceros, Coscinodiscus, Dactyliosolen, Detonula, Dictyocha, Dinophysis, Diploneis, Ditylum, Entomoneis, Guinardia, Gyrodinium, Gyrosigma, Hemiaulus, Leptocylindrus, Leptolyngbya, Lithodesmium, Melosira, Mesodinium, Meuniera, Microcystis, Neocalyptrella, Nitzschia, Noctiluca, Paralia, Peridinium, Planktolyngbya, Pleurosigma, Polykrikos, Proboscia, Prorocentrum, Protoperidinium, Pseudo-nitzschia, Pseudosolenia, Pterosperma, Raphidiopsis, Rhizosolenia, Scrippsiella, Skeletonema, Stephanopyxis, Surirella, Synedra, Thalassionema, Thalassiosira, Torodinium, Trieres, and Tripos.
Subgenera: Chaetoceros (Hyalochaeta), and Chaetoceros (Phaoceros).
Species: Akashiwo sanguínea, Alexandrium fraterculus, Asterionellopsis guyunusae, Aulacoseira granulata, Bacillaria paxillifera, Binuclearia lauterbornii, Campylosira cymbelliformis, Cerataulina bicornis, Ceratoneis closterium, Chaetoceros subtilis, Coscinodiscus wailesii, Dactyliosolen fragilissimus, Detonula pumila, Dictyocha fibula, Dinophysis acuminata, Dinophysis caudata, Ditylum brightwellii, Guinardia delicatula, Guinardia flaccida, Gyrodinium fusus, Leptocylindrus danicus, Leptocylindrus minimus, Melosira moniliformis, Meuniera membranacea, Neocalyptrella robusta, Noctiluca scintillans, Paralia sulcata, Peridinium quinquecorne, Polykrikos schwartzii, Prorocentrum compressum, Prorocentrum cordatum, Prorocentrum micans, Prorocentrum scutellum, Pseudosolenia calcar-avis, Rhizosolenia setigera, Scrippsiella trochoidea, Skeletonema costatum, Skeletonema tropicum, Stephanopyxis turris, Thalassionema nitzschioides, Trieres chinensis, Trieres mobiliensis, Tripos furca, and Tripos muelleri.
2.7.2 Micro- and mesozooplankton (dataset II)
Taxonomic ranks.
Kingdom: Animalia.
Phyla: Annelida, Arthropoda, Chaetognatha, Chordata, Cnidaria, Ctenophora, Echinodermata, Mollusca, Phoronida, and Rotifera.
Class: Appendicularia, Bivalvia, Branchiopoda, Gastropoda, Hexanauplia, Hydrozoa, Ichthyostraca, Malacostraca, Ostracoda, Polychaeta, Sagittoidea, and Thaliacea.
Orders: Amphipoda, Anomopoda, Aphragmophora, Arguloida, Calanoida, Copelata, Ctenopoda, Cumacea, Cyclopoida, Decapoda, Euphausiacea, Harpacticoida, Isopoda, Mysida, Onychopoda, Salpida, Sessilia, Siphonophorae, Siphonostomatoida, and Tanaidacea.
Families: Acartiidae, Balanidae, Bosminidae, Calanidae, Centropagidae, Chydoridae, Clausocalanidae, Corycaeidae, Cyclopidae, Daphniidae, Diaptomidae, Ditrichocorycaeus, Ectinosomatidae, Ergasilidae, Eurycercidae, Heterorhabdidae, Ilyocryptidae, Kalliapseudidae, Lubbockiidae, Luciferidae, Macrothricidae, Miraciidae, Moinidae, Oikopleuridae, Oithonidae, Oncaeidae, Onychocorycaeus, Paracalanidae, Peltidiidae, Podonidae, Pontellidae, Pseudodiaptomidae, Sagittidae, Sapphirinidae, Scolecitrichidae, Sididae, Subeucalanidae, Tachidiidae, and Temoridae.
Genera: Acanthocyclops, Acartia, Alona, Amphibalanus, Argyrodiaptomus, Belzebub, Biapertura, Boeckella, Bosmina, Bosminopsis, Calanoides, Calanus, Camptocercus, Centropages, Ceriodaphnia, Chydorus, Clausocalanus, Clytemnestra, Corycaeus, Ctenocalanus, Daphnia, Diaphanosoma, Ephemeroporus, Ergasilus, Eucyclops, Euryalona, Euterpina, Evadne, Flaccisagitta, Halicyclops, Heterorhabdus, Ilyocryptus, Kalliapseudes, Labidocera, Lubbockia, Macrosetella, Macrothrix, Mecynocera, Mesocyclops, Metacyclops, Microsetella, Moina, Nannocalanus, Notoalona, Notodiaptomus, Oikopleura, Oithona, Oncaea, Paracalanus, Parasagitta, Parvocalanus, Penilia, Pleopis, Podon, Pontellopsis, Pseudevadne, Pseudodiaptomus, Pseudosagitta, Pseudosida, Sapphirina, Scolecithrix, Simocephalus, Subeucalanus, Temora, and Undinula.
Species: Acanthocyclops robustus robustos, Acartia (Acanthacartia) tonsa, Acartia (Odontacartia) lilljeborgi, Alona costata, Alona monacantha, Amphibalanus improvisus, Argyrodiaptomus denticulatus, Biapertura affinis, Boeckella bergi, Bosmina (Bosmina) longirostris, Bosminopsis deitersi, Calanoides carinatus, Camptocercus australis, Centropages furcatus, Ceriodaphnia cornuta, Ceriodaphnia dubia, Chydorus eurynotus, Chydorus parvireticulatus, Chydorus sphaericus, Clausocalanus furcatus, Clausocalanus parapergens, Corycaeus speciosus, Ctenocalanus citer, Ctenocalanus vanus, Daphnia (Daphnia) ambigua, Diaphanosoma brachyurum, Diaphanosoma brevireme, Diaphanosoma fluviatile, Diaphanosoma spinulosum, Ditrichocorycaeus amazonicus, Eucyclops ensifer, Euryalona orientalis, Euterpina acutifrons, Evadne nordmanni, Flaccisagitta enflata, Goniopsyllus rostratus, Heterorhabdus papilliger, Ilyocryptus spinifer, Labidocera fluviatilis, Lubbockia squillimana, Macrosetella gracilis, Macrothrix spinosa, Mecynocera clausi, Mesocyclops longisetus longisetus, Metacyclops mendocinus mendocinus, Microsetella norvegica, Microsetella rosea, Moina micrura, Moina minuta, Nannocalanus minor, Notoalona sculpta, Notodiaptomus incompositus, Oithona nana, Oithona oswaldocruzi, Oithona plumifera, Oithona similis, Oithona simplex, Oncaea curvata, Oncaea lacinia, Oncaea mediterranea, Oncaea venusta, Oncaea waldemari, Onychocorycaeus giesbrechti, Paracalanus aculeatus, Paracalanus indicus, Paracalanus parvus, Paracalanus quasimodo, Parasagitta tenuis, Parvocalanus crassirostris, Penilia avirostris, Pleopis polyphemoides, Pleopis schmackeri, Pontellopsis brevis, Pseudevadne tergestina, Pseudodiaptomus richardi, Pseudosagitta lyra, Pseudosida bidentata, Scolecithrix danae, Simocephalus latirostris, Simocephalus serrulatus, Simocephalus vetulus, Subeucalanus pileatus, Temora stylifera, Temora turbinata, and Undinula vulgaris.
2.7.3 Ichthyoplankton (dataset III)
Taxonomic ranks.
Kingdom: Animalia.
Phylum: Chordata.
Class: Actinopterygii.
Orders: Anguilliformes, Atheriniformes, Beloniformes, Characiformes, Clupeiformes, Cyprinodontiformes, Elopiformes, Gobiesociformes, Perciformes, Pleuronectiformes, Siluriformes, and Syngnathiformes.
Families: Achiridae, Anablepidae, Atherinidae, Atherinopsidae, Blenniidae, Carangidae, Characidae, Clupeidae, Engraulidae, Gerreidae, Gobiesocidae, Gobiidae, Hemiramphidae, Mugilidae, Paralichthyidae, Pimelodidae, Poeciliidae, Sciaenidae, Stromateidae, Syngnathidae, and Trichiuridae.
Genera: Anchoa, Atherinella, Blennius, Brevoortia, Catathyridium, Cynoscion, Engraulis, Eucinostomus, Gobiesox, Gobionellus, Gobiosoma, Hyporhamphus, Jenynsia, Lycengraulis, Macrodon, Menticirrhus, Micropogonias, Mugil, Odontesthes, Paralichthys, Paralonchurus, Parapimelodus, Parona, Peprilus, Poecilia, Ramnogaster, Syngnathus, Trachinotus, and Trichiurus.
Species: Anchoa marinii, Brevoortia pectinata, Catathyridium garmani, Catathyridium jenynsii, Engraulis anchoita, Eucinostomus gula, Eucinostomus melanopterus, Gobiesox strumosus, Gobionellus oceanicus, Gobiosoma parri, Hyporhamphus roberti, Lycengraulis grossidens, Macrodon atricauda, Menticirrhus americanus, Micropogonias furnieri, Mugil curema, Mugil liza, Paralichthys orbignyanus, Paralonchurus brasiliensis, Parapimelodus nigribarbis, Parona signata, Poecilia vivipara, Ramnagaster arcuata, Syngnathus folletti, Trachinotus goodei, and Trichiurus lepturus.
2.7.4 Submerged aquatic vegetation (dataset IV)
Taxonomic ranks.
Kingdom: Plantae.
Subkingdoms: Biliphyta and Viridiplantae.
Division: Chlorophyta, Rhodophyta, and Tracheophyta.
Class: Florideophyceae, Magnoliopsida, and Ulvophyceae.
Orders: Acrochaetiales, Alismatales, Ceramiales, Cladophorales, and Ulvales.
Families: Acrochaetiaceae, Cladophoraceae, Rhodomelaceae, Ruppiaceae, and Ulvaceae.
Genera: Acrochaetium, Chaetomorpha, Cladophora, Polysiphonia, Rhizoclonium, Ruppia, and Ulva.
Species: Ruppia maritima (common name = widgeon grass), Chaetomorpha antennina, Chaetomorpha sp., Cladophora spp., Rhizoclonium riparium, Ulva clathrata, Ulva compressa, Ulva intestinalis, Ulva ramulosa, and Ulva sp.
2.7.5 Benthic macrofauna (dataset V)
Taxonomic ranks.
Kingdom: Animalia.
Phyla: Annelida, Arthropoda, and Mollusca.
Class: Bivalvia, Gastropoda, Malacostraca, and Polychaeta.
Orders: Cumacea, Isopoda, Littorinimorpha, Myida, Phyllodocida, and Tanaidacea.
Families: Capitellidae, Cochliopidae, Corbulidae, Diastylidae, Hyssuridae, Kalliapseudidae, Munnidae, Nephtyidae, Nereididae, Sphaeromatidae, and Tanaididae.
Genera: Alitta, Diastylis, Erodona, Heleobia, Heteromastus, Kupellonura, Laeonereis, Monokalliapseudes, Nephtys, Sinelobus, Sphaeromopsis, and Uromunna.
Species: Alitta succinea, Diastylis sympterygiae, Erodona mactroides, Heleobia australis, Heleobia charruana, Heteromastus similis, Laeonereis culveri, Monokalliapseudes schubarti, Nephtys fluviatilis, Sinelobus stanfordi, Sphaeromopsis mourei, and Uromunna peterseni.
2.7.6 Pink shrimp Penaeus paulensis (dataset VI)
Taxonomic ranks.
Kingdom: Animalia.
Phylum: Arthropoda.
Class: Malacostraca.
Order: Decapoda.
Family: Penaeidae.
Genus: Penaeus.
Species: Penaeus paulensis.
Common name: pink shrimp.
2.7.7 Fish assemblages (dataset VII)
Taxonomic ranks.
Kingdom: Animalia.
Phylum: Chordata.
Class: Actinopterygii.
Orders: Albuliformes, Anguilliformes, Atheriniformes, Batrachoidiformes, Beloniformes, Characiformes, Clupeiformes, Cyprinodontiformes, Elopiformes, Gobiesociformes, Perciformes, Pleuronectiformes, Scorpaeniformes, Siluriformes, Syngnathiformes, and Tetraodontiformes.
Families: Achiridae, Albulidae, Anablepidae, Ariidae, Atherinidae, Atherinopsidae, Batrachoididae, Callichthyidae, Carangidae, Characidae, Cichlidae, Clupeidae, Curimatidae, Cynoglossidae, Elopidae, Engraulidae, Erythrinidae, Gerreidae, Gobiesocidae, Gobiidae, Haemulidae, Hemiramphidae, Heptapteridae, Loricariidae, Monacanthidae, Mugilidae, Ophichthidae, Paralichthyidae, Percophidae, Pimelodidae, Pleuronectidae, Poeciliidae, Pomacentridae, Pomatomidae, Sciaenidae, Serranidae, Syngnathidae, Tetraodontidae, Trichiuridae, and Triglidae.
Genera: Abudefduf, Albula, Anchoa, Astyanax, Atherinella, Bathygobius, Brevoortia, Bryconamericus, Caranx, Catathyridium, Charax, Cheirodon, Chloroscombrus, Cichlasoma, Citharichthys, Cnesterodon, Corydoras, Crenicichla, Ctenogobius, Cyanocharax, Cynoscion, Cyphocharax, Diapterus, Elops, Engraulis, Epinephelus, Eucinostomus, Genidens, Geophagus, Gobiesox, Gobionellus, Gymnogeophagus, Harengula, Hemiramphus, Hippocampus, Hoplias, Hyphessobrycon, Hyporhamphus, Jenynsia, Lagocephalus, Loricariichthys, Lycengraulis, Macrodon, Macropsobrycon, Menticirrhus, Micropogonias, Mugil, Odontesthes, Oligoplites, Oligosarcus, Oncopterus, Ophichthus, Opisthonema, Orthopristis, Paralichthys, Paralonchurus, Parapimelodus, Percophis, Phalloceros, Phalloptychus, Phalloptychus, Pimelodella, Pimelodus, Platanichthys, Poecilia, Pogonias, Pomadasys, Pomatomus, Porichthys, Prionotus, Ramnogaster, Rhamdia, Sardinella, Selene, Stellifer, Stephanolepis, Symphurus, Syngnathus, Trachinotus, Trichiurus, Ulaema, and Umbrina.
Species: Abudefduf saxatilis, Albula vulpes, Amoya gracilis, Anchoa marinii, Astyanax dissensus, Astyanax eigenmanniorum, Astyanax fasciatus, Astyanax henseli, Astyanax lacustris, Atherinella brasiliensis, Bathygobius soporator, Brevoortia pectinata, Bryconamericus iheringii, Caranx hippos, Caranx latus, Catathyridium garmani, Charax stenopterus, Cheirodon ibicuhiensis, Cheirodon interruptus, Chloroscombrus chrysurus, Cichlasoma portalegrense, Citharichthys spilopterus, Cnesterodon decemmaculatus, Corydoras paleatus, Crenicichla lepidota, Ctenogobius shufeldti, Cyanocharax alburnus, Cynoscion leiarchus, Cynoscion striatus, Cyphocharax saladensis, Cyphocharax voga, Diapterus rhombeus, Elops saurus, Engraulis anchoita, Eucinostomus argenteus, Eucinostomus gula, Eucinostomus melanopterus, Genidens barbus, Genidens genidens, Genidens planifrons, Geophagus brasiliensis, Gobiesox strumosus, Gobionellus oceanicus, Gymnogeophagus gymnogenys, Harengula clupeola, Hemiramphus brasiliensis, Hippocampus patagonicus, Hoplias malabaricus, Hyphessobrycon anisitsi, Hyphessobrycon bifasciatus, Hyphessobrycon boulengeri, Hyphessobrycon igneus, Hyphessobrycon luetkenii, Hyphessobrycon meridionalis, Hyphessobrycon reticulatus, Hyphessobrycon togoi, Hyporhamphus unifasciatus, Jenynsia multidentata, Lagocephalus laevigatus, Loricariichthys anus, Lycengraulis grossidens, Macrodon ancylodon, Macrodon atricauda, Macropsobrycon uruguayanae, Menticirrhus americanus, Menticirrhus littoralis, Micropogonias furnieri, Mugil brevirostris, Mugil curema, Mugil liza, Odontesthes argentinensis, Odontesthes bonariensis, Odontesthes perugiae, Oligoplites saliens, Oligoplites saurus, Oligosarcus jenynsii, Oligosarcus robustus, Oncopterus darwinii, Ophichthus gomesii, Opisthonema oglinum, Orthopristis ruber, Paralichthys orbignyanus, Paralonchurus brasiliensis, Parapimelodus nigribarbis, Percophis brasiliensis, Phalloceros caudimaculatus, Phalloptychus eigenmanni, Phalloptychus januarius, Pimelodella australis, Pimelodus maculatus, Platanichthys platana, Poecilia vivipara, Pogonias courbina, Pomadasys corvinaeformis, Pomatomus saltatrix, Porichthys porosissimus, Prionotus punctatus, Ramnogaster arcuata, Rhamdia quelen, Sardinella brasiliensis, Selene setapinnis, Selene vomer, Stellifer brasiliensis, Stellifer rastrifer, Stellifer stellifer, Stephanolepis hispidus, Stephanolepis setifer, Symphurus jenynsi, Syngnathus folletti, Trachinotus carolinus, Trachinotus falcatus, Trachinotus goodei, Trachinotus marginatus, Trichiurus lepturus, Ulaema lefroyi, and Umbrina canosai.
2.7.8 Lahille's bottlenose dolphin Tursiops truncatus gephyreus (dataset VIII)
Taxonomic ranks.
Kingdom: Animalia.
Phylum: Chordata.
Class: Mammalia.
Order: Cetartiodactyla.
Family: Delphinidae.
Genus: Tursiops.
Species: Tursiops truncatus gephyreus.
Common name: Lahille's bottlenose dolphins.
2.8 Technical validation
All datasets have reliable sampling properties (same sampling methodology over time), have been thoroughly checked, have broad temporal and taxonomic coverage, and are ready to use for analyses (accompanied with metadata information). The data management, including the data validation process, consisted of (i) data acquisition and ecological validation, (ii) taxonomic validation, and (iii) structural validation:
- (i)
All data collection and ecological validation steps were carried out by the researchers responsible for the datasets (see quality assurance and control in Sect. 2.3).
- (ii)
Taxonomic nomenclature control was performed through the taxon match tool of the World Register of Marine Species (WoRMS, 2021), an authoritative and comprehensive list of marine organisms' taxonomy edited and reviewed by an international team of more than 240 taxonomic editors worldwide. Every species has a unique identifier known as a life sciences identifier (LSID), a persistent and globally unique identifier. This identifier links the species name to an internationally accepted standardized name and associated taxonomic information and also redirects to the most accurate information on the species taxonomy (e.g., accepted names and synonyms).
- (iii)
Prior to publication, all datasets' technical information has been individually reviewed regarding the use of the DwC terms and taxonomic validity during upload in the Integrated Publishing Toolkit (IPT) provided by the SiBBr and subsequent GBIF registration (datasets were validated by the Data Validator tool available from the GBIF). The Darwin Core was standardized according to the practices recommended by the TDWG guidelines (https://dwc.tdwg.org/terms/, last access: 10 October 2020). Sampling dates were formatted according to the ISO 8601 standard (i.e., YYYY-MM-DD). All files are available in unicode (UTF-8) format.
The LTER-PLEA's database policy follows the best practices of open data principles by releasing validated datasets on primary biodiversity and associated environmental data. The datasets were published in the GBIF repository through the Integrated Publishing Toolkit (IPT) provided by the Brazilian node SiBBr and can be accessed in the GBIF repository (Table 3). This publication refers to the most recent dataset published in the IPT. Monitoring is currently still being carried out, and the database will be updated and published every 4 years. All datasets presented here are identified by unique persistent identifiers such as digital object identifiers (DOIs) and are published under the Creative Commons Attribution-NonCommercial 4.0 International License (CC-BY-NC). Therefore, all datasets must be cited when used in scientific papers, presentations, reports, or any other by-product generated by researchers, governmental agencies, and the general public. Furthermore, it is desirable that the LTER-PLEA be included in the acknowledgements. When referring to the LTER-PLEA's database or sampling strategies and methodologies, please cite the present paper. The custodian of all the information collected is the Oceanography Institute of the Federal University of Rio Grande.
The LTER-PLEA's database is the first publicly available long-term database describing the abundance and composition of several components of planktonic, benthic, and pelagic biota from protists to mammals, associated with environmental data in an estuarine coastal system of South America. The LTER-PLEA's database has been the basis for several studies that investigate estuarine and coastal dynamics over time and has provided insights on the impacts of major anthropogenic and natural drivers, particularly the remote climate phenomena ENSO, across distinct taxonomic groups and trophic levels (Odebrecht et al., 2010, 2017).
The LTER-PLEA's database enabled the comprehension of the magnitude and drivers of short- and long-term changes in the abundance and composition of phytoplankton (Haraguchi et al., 2015), submerged aquatic vegetation (Copertino and Seeliger, 2010; Lanari and Copertino, 2017), benthic macrofauna (Collin et al., 2007, 2010), micro- and mesozooplankton assemblages (Muxagata et al., 2012; Teixeira-Amaral et al., 2017), the most relevant ichthyoplankton species (Bruno and Muelbert, 2009; Costa et al., 2013), fish fauna (Garcia et al., 2001, 2003, 2004), pink shrimp, and Lahille's bottlenose dolphin population parameters (Fruet et al., 2011, 2015; Genoves et al., 2018, 2020). All this information has enabled the understanding of the dynamics of ichthyoplankton transport and recruitment into the estuary (Costa and Muelbert, 2016; Franzen et al., 2019) and the influence of environmental dynamics on the health of fish larvae (Gouveia et al., 2015; Salvador and Muelbert, 2019); the influence of climatic and local factors on fish abundance, diversity, and trophic organization (Garcia et al., 2003, 2012, 2017; Possamai et al., 2018); the evaluation of the secondary production of copepods and its main contributors (Muxagata et al., 2012; Teixeira-Amaral et al., 2017); occurrence of potentially harmful microalgae groups (e.g., cyanobacteria and dinoflagellates) (Haraguchi et al., 2015); diatom accumulation in surf zone influenced by drastic events like mud deposition freshwater output (Odebrecht et al., 2010, 2013); phase shifts in the SAV (Copertino and Seeliger, 2010; Lanari and Copertino, 2017); the importance of the main nursery grounds for commercial species (D'Incao, 1991; Haimovici and Cardoso, 2017); overfishing impacts through analysis of Lahille's bottlenose dolphin (Fruet et al., 2011, 2014; Secchi et al., 2017); assessments of the conservation status and adaptive capacity and resilience of estuarine and marine organisms to anthropogenic changes and global warming (Bernardino et al., 2015; Copertino et al., 2016); among other relevant ecological processes.
ILTER datasets have subsidized meta-analyses of multidecadal biodiversity trends, hence corroborating the importance of long-term monitoring programs to offer insights on changes in natural systems (e.g., Pilotto et al., 2020). In general, long-term biodiversity data have been biased towards few taxonomic groups, and there is a lack of associated environmental data, hindering the understanding of the drivers of detected changes (Pilotto et al., 2020). Our database thus provides a comprehensive view of the biota's spatio-temporal dynamics and its environmental drivers in a subtropical coastal marine system in the southwestern Atlantic Ocean. Considering the over-representation of temperate regions in estuarine biodiversity and functioning studies (e.g., Vieillard et al., 2020), it allows for testing for generalizations of previous findings across distinct biogeographical areas.
The LTER-PLEA is one of the 115 globally distributed coastal and marine ILTER sites (ILTER-CMS), a network that provides several opportunities to study and monitor these ecosystems. ILTER-CMS constitutes an observation platform for the Global Ocean Observing System (GOOS)-defined essential ocean variables (EOVs) and several regional and global programs (Muelbert et al., 2019). Comparisons of our datasets obtained in the Southern Hemisphere with other estuaries worldwide would contribute to broaden our understanding of the role of the distinct signals (human versus climatic) in the biodiversity and functioning of these ecosystems (Paerl et al., 2015).
Our datasets can also contribute to analyses of emergent environmental issues over large temporal scales and geographic areas such as harmful algal blooms (Lyons et al., 2014) and overfishing (Brett et al., 2020). On a global scale, the LTER-PLEA's long-term data have already been supplied to analyses of range shifts in species distributions and abundance driven by climate change (Hastings et al., 2020). Despite the wide range of variables monitored, we acknowledge that several components that are key to assessing ecosystem quality are missing from our dataset. We have plans to monitor contaminants and heterotrophic prokaryotes in the near future. The environmental data such as water temperature and nutrient concentration time series may foster assessments of global warming and nutrient pollution in coastal marine systems. Despite the coastal nutrient pollution reported worldwide, few data are available for tropical and subtropical estuarine systems (Vieillard et al., 2020), and our data may help to fulfill this knowledge gap.
The sustainable use of ecosystem services can only be devised on a solid scientific basis (Carstensen, 2014). Thus, the information about the biological and physical properties of the system could be also used towards an integrative, interdisciplinary, and transversal approach, which may better link the estuary, coastal zone, and the ocean to their ecosystem services. Furthermore, such high-quality information adds to conservation, management, and restoration efforts of coastal and estuarine ecosystems (Costa et al., 2016), contributing to recommendations for public policies at local, national, and global levels.
MC and ML conceived the data paper. VML, ML, and MC wrote the manuscript. MC, EM, PCOVdA, JHM, FCD, ERS, JPV, AMG, AC, and CO coordinated the monitoring studies; provided metadata and data; and reviewed the information on occurrence, abundance, and taxonomic status of the species and abiotic data. VML formatted the data and published the LTER-PLEA's database in GBIF. All authors commented on the paper and contributed to the quality check.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We would like to thank Ulrich Seeliger, who was the creator of LTER-PLEA and coordinated the program from 1999 to 2010. The Brazilian Long-Term Ecological Research Program has been supported by the MCTI (Ministério de Ciência, Tecnologia e Inovação) and the Brazilian governmental funding agencies. The program also thanks the many laboratory technicians and undergraduate and graduate students who contributed to data sampling and organization.
This research has been supported by the Brazilian governmental funding agencies CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico; CNPq Proc. 520188/98-5, CNPq Proc. 558230/2009-1, CNPq Proc. 403805/2012-0, CNPq/CAPES/FAPs/BC – Fundo Newton/PELD no. 15/2016, CNPq/MCTI/CONFAP-FAPs/PELD no. 21/2020, Proc. 442206/2020-8), CAPES (Coordenação de Aperfeiçoamento Pessoal de Nivel Superior), and FAPERGS (Fundação de Amparo à Pesquisa do Rio Grande do Sul) as well as the Newton Fund (British Council) and the Organization for the Conservation of South American Aquatic Mammals (YAQUPACHA e.V., Nuremberg Zoo).
This paper was edited by François G. Schmitt and reviewed by Jacob Carstensen and one anonymous referee.
Abreu, P. C., Bergesch, M., Proença, L. A., Garcia, C. A. E., and Odebrecht, C.: Short-and long-term chlorophyll a variability in the shallow microtidal Patos Lagoon estuary, Southern Brazil, Estuar. Coast., 33, 554–569, https://doi.org/10.1007/s12237-009-9181-9, 2010.
Abreu, P. C., Marangoni, J., and Odebrecht, C.: So close, so far: differences in long-term chlorophyll a variability in three nearby estuarine-coastal stations, Mar. Biol. Res., 13, 1–13, https://doi.org/10.1080/17451000.2016.1189081, 2016.
Amaral, A. C. Z. and Nonato, E. F. (Eds.): Annelida Polychaeta. Características glossário e chaves para famílias e gêneros da costa brasileira, Editora Unicamp, Brazil, ISBN 85-268-0375-1, 1996.
Balech, E.: Los dinoflagelados del Atlántico sudoccidental, Publ. Espec. Inst. Esp. Oceanogr., 1, 1–310, http://hdl.handle.net/10508/993 (last access: 25 March 2019), 1988.
Baumgarten, M. G. Z. and Niencheski, L. F. H.: A coluna sedimentar como reservatório e fonte de nutrientes em enseadas estuarinas, Trop. Oceanogr., 38, 88–155, https://doi.org/10.5914/tropocean.v38i1.5163, 2010.
Bergesch, M., Garcia, M., and Odebrecht, C.: Diversity and morphology of Skeletonema species in Southern Brazil, Southwestern Atlantic Ocean, J. Phycol., 45, 1348–1352, https://doi.org/10.1111/j.1529-8817.2009.00743.x, 2009.
Bernardino, A. F., Netto, S. A., Pagliosa, P. R., Barros, F., Christofoletti, R. A., Rosa Filho, J. S., Colling, A., and Lana, P. C.: Predicting ecological changes on benthic estuarine assemblages through decadal climate trends along Brazilian Marine Ecoregions, Estuar. Coast. Shelf S., 166, 74–82, https://doi.org/10.1016/j.ecss.2015.05.021, 2015.
Biodiversity Information Standards (TDWG): Darwin Core Terms: A quick reference guide, http://www.tdwg.org/, last access: 10 October 2020.
Björnberg, T. K. S.: Copepoda, in: Atlas del Zooplancton del Atlántico Sudoccidental y métodos de trabajo con el zooplancton marino, edited by: Boltovskoy, D., Pub. Esp. INIDEP, Mar del Plata, Argentina, 587–679, 1981.
Boltovskoy, D. (Ed.): Atlas Del Zooplancton Del Atlantico Sudoccidental Y Metodos De Trabajo Con El Zooplancton Marino, INIDEP, Mar del Plata, Argentina, 1981.
Boltovskoy, D. (Ed.): South Atlantic Zooplankton, vol. 1–2, Backhuys Publishers, Leiden, ISBN 9057820358 9789057820359, 1999.
Bradford-Grieve, J. M.: Copepoda – Sub-Order: Calanoida – Family: Acartiidae – Genus: Acartia, in: Fiches d'identification du Zooplancton No. 12, ICES, Copenhagen, 1–19, ISSN 1019-1097, 1999.
Brett, A., Leape, J., Abbott, M., Sakaguchi, H., Cao, L., Chand, K., Golbuu, Y., Martin, T. J., Mayorga, J., and Myksvoll, M. S.: Ocean data need a sea change to help navigate the warming world, Nature, 582, 181–183, https://doi.org/10.1038/d41586-020-01668-z, 2020.
Bruno, M. A. and Muelbert, J. H.: Distribuição espacial e variações temporais da abundância de ovos e larvas de Micropogonias furnieri no estuário da Lagoa dos Patos: registros históricos e forçantes ambientais, Atlântica, 31, 51–68, https://doi.org/10.5088/atl.2009.31.1.51, 2009.
Buckup, L. and Bond-Buckup, G.: Os Crustáceos Do Rio Grande Do Sul, Editora da Universidade Federal do Rio Grande do Sul, Brazil, ISBN 9788570255020, 1999.
Carstensen, J.: Need for monitoring and maintaining sustainable marine ecosystem services, Front. Mar. Sci., 1, 1–4, https://doi.org/10.3389/fmars.2014.00033, 2014.
Castello, J. P. and Möller Jr., O. O.: On the relationship between rainfall and shrimp production in the estuary of the Patos Lagoon (RS-Brazil), Atlântica, 3, 67–74, 1978.
Christian, R. R. and Mazzilli, S.: Defining the coast and sentinel ecosystems for coastal observations of global change, Hydrobiologia, 577, 55–70, https://doi.org/10.1007/978-1-4020-6008-3_6, 2007.
Colling, L. A. and Cavalca Bom, F.: Temporal data series of Benthic macrofauna abundance and composition from the Patos Lagoon estuary, GBIF [data set], https://doi.org/10.15468/lsoc2v, 2020.
Colling, L. A., Bemvenuti, C. E., and Gandra, M. S.: Seasonal variability on the structure of sublittoral macrozoobenthic association in the Patos Lagoon estuary, southern Brazil, Iheringia, Ser. Zool., 97, 257–262, https://doi.org/10.1590/S0073-47212007000300007, 2007.
Colling, L. A., Bemvenuti, C. E., and Pinotti, R. M.: Temporal variability of the bivalve Erodona mactroides BOSC, 1802 during and after the El Niño phenomenon (2002/2003) in a subtropical lagoon, southern Brazil, Acta Limnol. Bras., 22, 410–423, https://doi.org/10.4322/actalb.2011.006, 2010.
CONCEA: Diretrizes da prática de eutanásia do CONCEA, Ministério da Ciência, Tecnologia e Inovação Conselho Nacional de Controle de Experimentação Animal, Brasil, 2013.
Copertino, M.: Dynamics of Submerged Aquatic Vegetation in the Patos Lagoon estuary, GBIF [data set], https://doi.org/10.15468/bjzlnb, 2020.
Copertino, M. and Seeliger, U.: Habitats de Ruppia maritima e de macroalgas, in: O estuário da Lagoa dos Patos: Um Século de Transformações, edited by: Seeliger, U. and Odebrecht, C., Editora FURG, Brazil, 89–98, ISBN 978-85-7566-144-4, 2010.
Copertino, M. S., Creed, J. C., Magalhães, K. M., Barros, K. D. S., Lanari, M. D. O., Arévalo, P. R., and Horta, P. A.: Monitoramento dos fundos vegetados submersos (pradarias submersas), in: Protocolos para o Monitoramento de Habitats Bentônicos Costeiros – Rede de Monitoramento de Habitats Bentônicos Costeiros – ReBentos, edited by: Turra, A. and Denadai, M., 1st edn., Instituto Oceanográfico da Universidade de São Paulo – IO/USP, Brazil, https://doi.org/10.7476/9788598729251, 2015.
Copertino, M. S., Creed, J. C., Lanari, M., Magalhães, K., Barros, K., Lana, P. C., Sordo, L., and Horta, P. A.: Seagrass and submerged aquatic vegetation (VAS) habitats off the coast of Brazil: state of knowledge, conservation and main threats, Braz. J. Oceanogr., 64, 53–80, https://doi.org/10.1590/S1679-875920161036064sp2, 2016.
Costa, M. D. and Muelbert, J. H.: Long-term assessment of temporal variability in spatial patterns of early life stages of fishes to facilitate estuarine conservation, Mar. Biol. Res., 13, 1–14, https://doi.org/10.1080/17451000.2016.1213397, 2016.
Costa, M. D., Muelbert, J. H., Moraes, L. E., Vieira, J. P., and Castello, J. P.: Estuarine early life stage habitat occupancy patterns of whitemouth croaker Micropogonias furnieri (Desmarest, 1830) from the Patos Lagoon, Brazil, Fish. Res., 160, 77–84, https://doi.org/10.1016/j.fishres.2013.10.025, 2013.
Costa, M. D., Possingham, H. P., and Muelbert, J. H.: Incorporating early life stages of fishes into estuarine spatial conservation planning, Aquat. Conserv., 26, 1013–1030, https://doi.org/10.1002/aqc.2584, 2016.
D'Incao, F.: Pesca e biologia de Penaeus paulensis na Lagoa dos Patos, RS, Atlântica, 13, 159–169, 1991.
De Pooter, D., Appeltans, W., Bailly, N., Bristol, S., Deneudt, K., Eliezer, M., Fujioka, E., Giorgetti, A., Goldstein, P., Lewis, M., Lipizer, M., Mackay, K., Marin, M., Moncoiffé, G., Nikolopoulou, S., Provoost, P., Rauch, S., Roubicek, A., Torres, C., van de Putte, A., Vandepitte, L., Vanhoorne, B., Vinci, M., Wambiji, N., Watts, D., Salas, E. K., and Hernandez, F.: Toward a new data standard for combined marine biological and environmental datasets-expanding OBIS beyond species occurrences, Biodivers. Data J., 5, 1–37, https://doi.org/10.3897/BDJ.5.e10989, 2017.
Doney, S. C. and Schimel, D.: Climate Change and Biogeochemical Impacts, eLS, https://doi.org/10.1002/9780470015902.a0003242.pub3, 2015.
Dreujou, E., Carrier-Belleau, C., Goldsmit, J., Fiorentino, D., Ben-Hamadou, R., Muelbert, J. H., Godbold, J. A., Daigle, R. M., and Beauchesne, D.: Holistic Environmental Approaches and Aichi Biodiversity Targets: accomplishments and perspectives for marine ecosystems, Peer J., 8, 1–22, https://doi.org/10.7717/peerj.8171, 2020.
Duffy, J. E., Amaral-Zettler, L. A., Fautin, D. G., Paulay, G., Rynearson, T. A., Sosik, H. M., and Stachowicz, J. J.: Envisioning a marine biodiversity observation network, Bioscience, 63, 350–361, https://doi.org/10.1525/bio.2013.63.5.8, 2013.
Dumont, L. F. C.: Ecology of the pink-shrimp Penaeus paulensis in Patos Lagoon estuary, GBIF [data set], https://doi.org/10.15468/ovayhc, 2020.
Fahay, M. P.: Guide to the early stages of marine fishes occurring in the western North Atlantic Ocean, Cape Hatteras to the southern Scotian Shelf, J. Northwest Atl. Fish. Sci., 4, 1–423, 1983.
Figueiredo, J. L. (Ed.): Manual De Peixes Marinhos Do Sudeste Do Brasil. I. Introdução. Cações, Raias E Quimeras, Museu de Zoologia da Universidade de São Paulo, Brazil, https://doi.org/10.5962/bhl.title.109986, 1977.
Figueiredo, J. L. and Menezes, N. A. (Eds.): Manual De Peixes Marinhos Do Sudeste Do Brasil. II. Teleostei (1), Museu de Zoologia da Universidade de São Paulo, Brazil, https://doi.org/10.5962/bhl.title.109986, 1978.
Figueiredo, J. L. and Menezes, N. A. (Eds.): Manual De Peixes Marinhos Do Sudeste Do Brasil. III. Teleostei (2) Museu de Zoologia da Universidade de São Paulo, Brazil, https://doi.org/10.5962/bhl.title.109986, 1980.
Figueiredo, J. L. and Menezes, N. A. (Eds.): Manual De Peixes Marinhos Do Sudeste Do Brasil. VI. Teleostei (5), Museu de Zoologia da Universidade de São Paulo, Brazil, 2000.
Fischer, L. G., Pereira, L. E. D., and Vieira, J. P. (Eds.): Peixes Estuarinos E Costeiros, Ecoscientia, Rio Grande, Brazil, ISBN 9788591209514, 2004.
Franco, A. D. O. D. R., They, N. H., Canani, L. G. D. C., Maggioni, R., and Odebrecht, C.: Asterionellopsis tropicalis (Bacillariophyceae): a new tropical species found in diatom accumulations, J. Phycol., 52, 888–895, https://doi.org/10.1111/jpy.12435, 2016.
Franzen, M. O., Fernandes, E. H. L., and Muelbert, J. H.: Influence of wind events on the transport of early stages of Micropogonias furnieri (Desmarest, 1823) to a subtropical estuary, Lat. Am. J. Aquat. Res., 47, 536–546, https://doi.org/10.3856/vol47-issue3-fulltext-15, 2019.
Froese, R. and Pauly, D.: FishBase, World Wide Web electronic publication, https://www.fishbase.org, last access: 14 April 2020.
Fruet, P. F., Secchi, E. R., Di Tullio, J. C., and Kinas, P. G.: Abundance of bottlenose dolphins, Tursiops truncatus (Cetacea: Delphinidae), inhabiting the Patos Lagoon estuary, southern Brazil: implications for conservation, Zoologia., 28, 23–30, https://doi.org/10.1590/S1984-46702011000100004, 2011.
Fruet, P. F., Secchi, E. R., Daura-Jorge, F., Vermeulen, E., Flores, P. A. C., Simões-Lopes, P. C., Genoves, R. C., Laporta, P., Di Tullio, J. C., Freitas, T. R. O., Rosa, L. D., Valiati, V. H., Beheregaray, L. B., and Möller, L. M.: Remarkably low genetic diversity and strong population structure in common bottlenose dolphins (Tursiops truncatus) from coastal waters of the Southwestern Atlantic Ocean, Conserv. Genet., 15, 879–895, https://doi.org/10.1007/s10592-014-0586-z, 2014.
Fruet, P. F., Genoves, R. C., Möller, L. M., Botta, S., and Secchi, E. R.: Using mark-recapture and stranding data to estimate reproductive traits in female bottlenose dolphins (Tursiops truncatus) of the Southwestern Atlantic Ocean, Mar. Biol., 162, 661–673, https://doi.org/10.1007/s00227-015-2613-0, 2015.
Garcia, A. M., Vieira, J. P., and Winemiller, K. O.: Dynamics of the shallow-water fish assemblage of the Patos Lagoon estuary (Brazil) during cold and warm ENSO episodes, J. Fish Biol., 59, 1218–1238, https://doi.org/10.1111/j.1095-8649.2001.tb00187.x, 2001.
Garcia, A. M., Vieira, J. P., and Winemiller, K. O.: Effects of 1997–1998 El Niño on the dynamics of the shallow-water fish assemblage of the Patos Lagoon Estuary (Brazil), Estuar. Coast. Shelf S., 57, 489–500, https://doi.org/10.1016/S0272-7714(02)00382-7, 2003.
Garcia, A. M., Vieira, J. P., Winemiller, K. O., and Grimm, A. M.: Comparison of 1982–1983 and 1997–1998 El Niño effects on the shallow-water fish assemblage of the Patos Lagoon estuary (Brazil), Estuaries, 27, 905–914, https://doi.org/10.1007/BF02803417, 2004.
Garcia, A. M., Vieira, J. P., Winemiller, K. O., Moraes, L. E., and Paes, E. T.: Factoring scales of spatial and temporal variation in fish abundance in a subtropical estuary, Mar. Ecol. Prog. Ser., 461, 121–135, https://doi.org/10.3354/meps09798, 2012.
Garcia, A. M., Winemiller, K. O., Hoeinghaus, D. J., Claudino, M. C., Bastos, R., Correa, F., Huckembeck, S., Vieira, J., Loebmann, D., Abreu, P. C., and Ducatti, C.: Hydrologic pulsing promotes spatial connectivity and food web subsidies in a subtropical coastal ecosystem, Mar. Ecol. Prog. Ser., 567, 17–28, https://doi.org/10.3354/meps12060, 2017.
Garcia, M. and Odebrecht, C.: Chave dicotômica ilustrada para a identificação de espécies de Thalassiosira cleve (diatomácea) no estuário da Lagoa dos Patos e área costeira adjacente (Rio Grande do Sul, Brasil), Biota Neotrop., 9, 239–253, https://doi.org/10.1590/S1676-06032009000200023, 2009.
Genoves, R. C., Fruet, P. F., Di Tullio, J. C., Möller, L. M., and Secchi, E. R.: Spatiotemporal use predicts social partitioning of bottlenose dolphins with strong home range overlap, Ecol. Evol., 8, 12597–12614, https://doi.org/10.1002/ece3.4681, 2018.
Genoves, R. C., Fruet, P. F., Botta, S., Beheregaray, L. B., Möller, L. M., and Secchi, E. R.: Fine-scale genetic structure in Lahille's bottlenose dolphins (Tursiops truncatus gephyreus) is associated with social structure and feeding ecology, Mar. Biol., 167, 1–16, https://doi.org/10.1007/s00227-019-3638-6, 2020.
Gouveia, G. R., Trindade, G. S., Nery, L. E. M., and Muelbert, J. H.: UVA and UVB penetration in the water column of a South West Atlantic warm temperate estuary and its effects on cells and fish larvae, Estuar. Coast., 38, 1147–1162, https://doi.org/10.1007/s12237-015-9996-5, 2015.
Gray, J. S. and Elliott, M. (Eds.): Ecology of Marine Sediments, 2nd edn., Oxford: Oxford University Press, EUA, ISBN 978-0-19-856901-5, 2009.
Grimm, A. M., Ferraz, S. E. T., and Gomes, J.: Precipitation anomalies in Southern Brazil associated with El Niño and La Niña events, J. Climate, 11, 2863–2880, https://doi.org/10.1175/1520-0442(1998)011<2863:PAISBA>2.0.CO;2, 1998.
Hagström, J. A., Granéli, E., Moreira, M. O., and Odebrecht, C.: Produção de ácido domóico e composição elementar de duas cepas multissérie de Pseudo-nitzschia, do NW e SW do Oceano Atlântico, crescendo em culturas quimiostáticas limitadas por fósforo ou nitrogênio, J. Plankton Res., 33, 297–308, 2011.
Haimovici, M. and Cardoso, L. G.: Long-term changes in the fisheries in the Patos Lagoon estuary and adjacent coastal waters in Southern Brazil, Mar. Biol. Res., 13, 135–150, https://doi.org/10.1080/17451000.2016.1228978, 2017.
Haimovici, M., Castello, J. P., and Abdallah, P. R.: Desenvolvimento da pesca industrial sediada em Rio Grande: uma visão histórica sob a ótica de atores privilegiados, in: A pesca marinha e estuarina no Brasil: estudos de caso, edited by: Haimovici, M., Andriguetto, J. M., and Sunye, P. S., Editora da FURG, Brazil, 17–28, ISBN 978-85-7566-335-6, 2014.
Halpern, B. S., Walbridge, S., Selkoe, K. A., Kappel, K. V., Micheli, F., D'agrosa, K., Bruno, J. F., Casey, K. S., Ebert, C., Fox, H. E., Fujita, R., Heinemann, D., Lenihan, H. S., Madin, E. M. P., Perry, M. T., Selig, E. R., Spalding, M., Steneck, R., and Watson, R.: A global map of human impact on marine ecosystems, Science, 319, 948–952, https://doi.org/10.1126/science.1149345, 2008.
Haraguchi, L., Carstensen, J., Abreu, P. C., and Odebrecht, C.: Long-term changes of the phytoplankton community and biomass in the subtropical shallow Patos Lagoon Estuary, Brazil, Estuar. Coast. Shelf S., 162, 76–87, https://doi.org/10.1016/j.ecss.2015.03.007, 2015.
Hastings, R. A., Rutterford, L. A., Freer, J. J., Collins, R. A., Simpson, S. D., and Genner, M. G.: Climate change drives pole ward increases and equator ward declines in marine species, Curr. Biol., 30, 1572–1577, https://doi.org/10.1016/j.cub.2020.02.043, 2020.
Hoppenrath, M., Elbrächter, M., and Drebes, G. (Eds.): Marine Phytoplankton, Kleine Senckenberg-Reihe, Band 49, Germany, ISBN 9783510613922, 2009.
Kalikoski, D. C. and Vasconcellos, M.: Case study of the technical, socio-economic and environmental conditions of small-scale fisheries in the estuary of Patos Lagoon, Brazil: a methodology for assessment, FAO Fisheries and Aquaculture, Circular C1075I, ISBN 978-92-5-107142-7, 2012.
Kennish, M. J. and Paerl, H. W. (Eds.): Coastal Lagoons: Critical Habitats of Environmental Change, CRC Press, EUA, ISBN 9781420088304, 2010.
Kjerfve, B.: Comparative oceanography of coastal lagoons, in: Estuarine variability, edited by: Wolfe, D. A., Academic Press, 63–81, https://doi.org/10.1016/B978-0-12-761890-6.50009-5, 1986.
Lanari, M. and Copertino, M.: Drift macroalgae in the Patos Lagoon Estuary (southern Brazil): effects of climate, hydrology and wind action on the onset and magnitude of blooms, Mar. Biol. Res., 13, 36–47, https://doi.org/10.1080/17451000.2016.1225957, 2017.
Lanari, M., Claudino, M. C., Garcia, A. M., and Copertino, M.: Changes in the elemental (C, N) and isotopic (δ13C, δ15N) composition of estuarine plants during diagenesis and implications for ecological studies, J. Exp. Mar. Biol. Ecol., 500, 46–54, https://doi.org/10.1016/j.jembe.2017.12.013, 2018.
Lang, W. H.: Larval development of shallow water barnacles of the carolinas (Cirripedia: Thoracica) with keys to naupliar stages, NOAA Technical Report NMFS, Circular 421, https://doi.org/10.5962/bhl.title.63163, 1979.
Lang, W. H.: Cirripedia: balanomorph nauplii of the NW Atlantic shores, Fiche 163, Fiches d'Identification du Zooplancton, Conseil International pour l'Exploration de la Mer (CIEM), Scotland, https://doi.org/10.17895/ices.pub.5148, 1980.
Lotze, H. K., Lenihan, H. S., Bourque, B. J., Bradbury, R. H., Cooke, R. G., Kay, M. C., Kidwell, S. M., Kirby, M. X., Peterson, C. H., and Jackson, J. B. C.: Depletion, degradation, and recovery potential of estuaries and coastal seas, Science, 312, 1806–1809, https://doi.org/10.1126/science.1128035, 2006.
Lyons, T. W., Reinhard, C. T., and Planavsky, N. J.: The rise of oxygen in Earth's early ocean and atmosphere, Nature, 506, 307–315, https://doi.org/10.1038/nature13068, 2014.
McLusky, D. S. and Elliott, M.: The Estuarine Ecosystem: Ecology, Threats and Management, OUP Oxford, EUA, https://doi.org/10.1093/acprof:oso/9780198525080.001.0001, 2004.
Menezes, N. A. and Figueiredo, J. L. (Eds.): Manual De Peixes Marinhos Do Sudeste Do Brasil. IV. Teleostei (3), Museu de Zoologia da Universidade de São Paulo, Brazil, 1980.
Menezes, N. A. and Figueiredo, J. L. (Eds.): Manual De Peixes Marinhos Do Sudeste Do Brasil. V. Teleostei (4) Museu de Zoologia da Universidade de São Paulo, Brazil, 1985.
Möller, O. O., Castaing, P., Salomon, J. C., and Lazure, P.: The influence of local and non-local forcing effects on the subtidal circulation of Patos Lagoon, Estuaries, 24, 297–311, https://doi.org/10.2307/1352953, 2001.
Moraes, L. E., Paes, E., Garcia, A. M., Moller Jr, O., and Vieira, J. P.: Delayed response of fish abundance to environmental changes: a novel multivariate time-lag approach, Mar. Ecol. Prog. Ser., 456, 159–168, https://doi.org/10.3354/meps09731, 2012.
Moser, H. G.: The Early Stages Of Fishes In The California Current Region, CalCOFI Atlas, EUA, ISBN 0-935868-82-8, 1996.
Muelbert, J. H.: Interannual variability of ichthyoplankton diversity in the Patos Lagoon estuary Southern Brazil, GBIF [data set], https://doi.org/10.15468/noeqwa, 2020.
Muelbert, J. H., Nidzieko, N. J., Acosta, A. T. R., Beaulieu, S. E., Bernardino, A. F., Boikova, E., Bornman, T. G., Cataletto, B., Deneudt, K., Eliason, E., Kraberg, A., Nakaoka, M., Pugnetti, A., Ragueneau, O., Scharfe, M., Soltwedel, T., Sosik, H. M., Stanisci, A., Stefanova, K., Stéphan, P., Stier, A., Wikner, J., and Zingone, A.: ILTER – The International Long-Term Ecological Research Network as a Platform for Global Coastal and Ocean Observation, Front. Mar. Sci., 6, 01–14, https://doi.org/10.3389/fmars.2019.00527, 2019.
Muxagata, E. and Teixeira-Amaral, P.: Continuous monitoring of the micro and mesozooplankton of the Patos Lagoon estuary and adjacent coastal area, GBIF [data set], https://doi.org/10.15468/1xkowr, 2020.
Muxagata, E., Amaral, W. J., and Barbosa, C. N.: Acartia tonsa production in the Patos Lagoon estuary, Brazil, ICES J. Mar. Sci., 69, 475–482, https://doi.org/10.1093/icesjms/fsr166, 2012.
Newton, A., Brito, A. C., Icely, J. D., Derolez, V., Clara, I., Angus, S., Schernewski, G., Inácio, M., Lillebø, A. I., Sousa, A. I., Béjaoui, B., Solidoro, C., Tosic, M., Cañedo-Argüelles, M., Yamamuro, M., Reizopoulou, S., Tseng, H.-C., Canu, D., Roselli, L., Maanan, M., Cristina, S., Ruiz-Fernández, A. C., de Lima, R. F., Kjerfve, B., Rubio-Cisneros, N., Pérez-Ruzafa, A., Marcos, C., Pastres, R., Pranovi, F., Snoussi, M., Turpie, J., Tuchkovenko, Y., Dyack, B., Brookes, J., Povilanskas, B., and Khokhlov, V.: Assessing, quantifying and valuing the ecosystem services of coastal lagoons, J. Nat. Conserv., 44, 50–65, https://doi.org/10.1016/j.jnc.2018.02.009, 2018.
Odebrecht, C. and Abreu, P. C.: Phytoplankton and water quality parameters in the Patos Lagoon estuary and adjacent marine coast, GBIF [data set], https://doi.org/10.15468/xmlvxm, 2020.
Odebrech, C. and Castello, J. P.: The Convergence Ecosystem in the Southwest Atlantic, in: Subtropical Convergence Environments: The coast and sea in the Southwestern Atlantic, edited by: Seeliger, U. and Kjerfve, B., Ecological Studies (Analysis and Synthesis), vol. 144, Springer, Berlin, 145–165, https://doi.org/10.1007/978-3-662-04482-7_12, 2001.
Odebrecht, C., Abreu, P. C., Bemvenuti, C. E., Copertino, M., Muelbert, J. H., Vieira, J. P., and Seeliger, U.: The Patos Lagoon Estuary: biotic responses to natural and anthropogenic impacts in the last decades (1979–2008), in: Coastal lagoons: critical habitats of environmental change, edited by: Kennish, M. J. and Paerl, H. W., CRC Press, EUA, 437–459, ISBN 9781420088304, 2010.
Odebrecht, C., Abreu, P. A., Bemvenuti, C. E., Colling, L. A., Copertino, M., Costa, C. S. B., Garcia, A. M., Marangoni, J. C., Moller, O. O., Muelbert, J. H., Vieira, J., and Seeliger, U.: O efeito de perturbações naturais e antrópicas na ecologia do Estuário da Lagoa dos Patos, in: PELD-CNPQ, dez anos do programa de pesquisas ecológicas de longa duração do Brasil: achados, lições e perspectivas, edited by: Tabarelli, M., Rocha, C. F. D., Romanowski, H. P., Rocha, O., and Lacerda, L. D., Editora Universitaria UFPE, Brazil, 223–248, ISBN 978-85-415-0329-7, 2013.
Odebrecht, C., Abreu, P. C., and Carstensen, J.: Retention time generates short-term phytoplankton blooms in a shallow microtidal subtropical estuary, Estuar. Coast. Shelf S., 162, 35–44, https://doi.org/10.1016/j.ecss.2015.03.004, 2015.
Odebrecht, C., Secchi, E. R., Abreu, P. C., Muelbert, J. H., and Uiblein, F.: Biota of the Patos Lagoon estuary and adjacent marine coast: long-term changes induced by natural and human-related factors, Mar. Biol. Res., 13, 3–8, https://doi.org/10.1080/17451000.2016.1258714, 2017.
Olivar, M. P., Moser, H. G., and Beckley, L. E.: Lanternfish larvae from the Agulhas current (SW Indian Ocean), Sci. Mar., 63, 101–120, https://doi.org/10.3989/scimar.1999.63n2101, 1999.
Paerl, H. W., Yin, K., and O'Brien, T. D.: SCOR Working Group 137: Global Patterns of Phytoplankton Dynamics in Coastal Ecosystems: An introduction to the special issue of Estuarine, Coastal and Shelf Science, Estuar. Coast. Shelf S., 162, 1–3, https://doi.org/10.1016/j.ecss.2015.07.011, 2015.
Pilotto, F., Kühn, I., Adrian, R., Alber, R., Alignier, A., Andrews, C., Bäck, J., Barbaro, L., Beaumont, D., Beenaerts, N., Benham, S., Boukal, D. S., Bretagnolle, V., Camatti, E., Canullo, R., Cardoso, P. G., Ens, B. J., Everaert, G., Evtimova, V., Feuchtmayr, H., García-González, R., García, D. G., Grandin, U., Gutowski, J. M., Hadar, L., Halada, L., Halassy, M., Hummel, H., Huttunen, K.-L., Jaroszewicz, B., Jensen, T. C., Kalivoda, H., Schmidt, I. K., Kröncke, I., Leinonen, R., Martinho, F., Meesenburg, H., Meyer, J., Minerbi, S., Monteith, D., Nikolov, B. P., Oro, D., Ozolinš, D., Padedda, B. M., Pallett, D., Pansera, M., Pardal, M. A., Petriccione, B., Pipan, T., Pöyry, J., Schäfer, S. M., Schaub, M., Schneider, S. C., Skuja, A., Soetaert, K., Sprige, G., Stanchev, R., Stockan, J. A., Stoll, S., Sundqvist, L., Thimonier, A., Van Hoey, G., Van Ryckegem, G., Visser, M. E., Vorhauser, S., and Haase, P.: Meta-analysis of multidecadal biodiversity trends in Europe, Nat. Commun., 11, 1–11, https://doi.org/10.1038/s41467-020-17171-y, 2020.
Possamai, B., Vieira, J. P., Grimm, A. M., and Garcia, A. M.: Temporal variability (1997-2015) of trophic fish guilds and its relationships with El Niño events in a subtropical estuary, Estuar. Coast. Shelf S., 202, 145–154, https://doi.org/10.1016/j.ecss.2017.12.019, 2018.
Richards, W. J. (Ed.): Early stages of Atlantic fishes. An Identification Guide for the Western Central North Atlantic, CRC Press, Boca Raton, Florida, EUA, https://doi.org/10.1201/9780203500217, 2005.
Rios, E. (Ed.): Compendium of Brazilian Sea Shells, Evan Graf, Brazil, ISBN 9788577271733, 2009.
Robertson, A. W. and Mechoso, C. R.: Interannual and Decadal Cycles in River Flows of Southeastern South America, J. Climate, 11, 2570–2581, https://doi.org/10.1175/1520-0442(1998)011<2570:IADCIR>2.0.CO;2, 1997.
Rose, M. (Ed.): Faune de France 26 – Copepodes pelagiques, Federation Francaise des Societes de Sciences Naturelles, Paris, France, https://doi.org/10.1038/132767a0, 1933.
Ruiz, G. M., Fofonoff, P. W., Carlton, J. T., Wonham, M. J., and Hines, A. H.: Invasion of coastal marine communities in North America: apparent patterns, processes, and biases, Annu. Rev. Ecol. Evol. S., 31, 481–531, https://doi.org/10.1146/annurev.ecolsys.31.1.481, 2000.
Salvador, N. L. A. and Muelbert, J. H.: Environmental Variability and Body Condition of Argentine Menhaden Larvae, Brevoortia pectinata (Jenyns, 1842), in: Estuarine and Coastal Waters, Estuar. Coast., 42, 1654–1661, https://doi.org/10.1007/s12237-019-00604-3, 2019.
Secchi, E. R., Botta, S., Wiegand, M. M., Lopez, L. A., Fruet, P. F., Genoves, R. C., and Di Tullio, J. C.: Long-term and gender-related variation in the feeding ecology of common bottlenose dolphins inhabiting a subtropical estuary and the adjacent marine coast in the western South Atlantic, Mar. Biol. Res., 13, 121–134, https://doi.org/10.1080/17451000.2016.1213398, 2017.
Secchi, E. R., Fruet, P., and Genoves, R. C.: Ecology of Lahille's bottlenose dolphin Tursiops truncatus gephyreus in the Patos Lagoon estuary and adjacent marine coast, GBIF [data set], https://doi.org/10.15468/4nh9ng, 2020.
Seeliger, U.: The Patos Lagoon Estuary, Brazil, in: Coastal marine ecosystems of Latin América, edited by: Seeliger, U. and Kjerfve, B., Springer, Berlin, Heidelberg, 167–183, ISBN 3540672281 9783540672289, 2001.
Seeliger, U. and Odebrecht, C. (Eds.): O Estuário da Lagoa dos Patos: um século de transformações, Editora da Furg, Brazil, ISBN 978-85-7566-144-4, 2010.
Steedman, H. F. V.: Aldehydes – 1, General and applied data on formaldehyde fixation and preservation of marine zooplankton, in: Monographs on oceanographic methodology, No. 4 – Zooplankton fixation and preservation, edited by: Steedman, H. F., UNESCO Press, Paris, 103–154, ISBN 92-3-101272-x, 1976.
Strickland, J. D. and Parsons, T. R.: A practical handbook of seawater analysis, B. Fish. Res. Board Can., 167, 1–309, https://doi.org/10.1002/iroh.19700550118, 1972.
Tanhua, T., Pouliquen, S., Hausman, J., Obrien, K., Bricher, P., Bruin, T., Buck, J. J. H., Burger, E. F., Carval, T., Casey, K. S., Diggs, S., Giorgetti, A., Glaves, H., Harscoat, V., Kinkade, D., Muelbert, J. H., Novellino, A., Pfeil, B., Pulsifer, P. L., Putte, A. V., Robinson, E., Schaap, D., Smirnov, A., Smith, N., Snowden, D., Spears, T., Stall, S., Tacoma, M., Thijsse, P., Tronstad, S., Vandenberghe, T., Wengren, M., Wyborn, L., and Zhao, Z.: Ocean FAIR Data Services, Front. Mar. Sci., 6, 1–17, https://doi.org/10.3389/fmars.2019.00440, 2019.
Teixeira-Amaral, P., Amaral, W. J. A., de Ortiz, D. O., Agostini, V. O., and Muxagata, E.: The mesozooplankton of the Patos Lagoon Estuary, Brazil: trends in community structure and secondary production, Mar. Biol. Res., 13, 48–61, https://doi.org/10.1080/17451000.2016.1248850, 2017.
Tomas, C. R. (Ed.): Marine Diatoms and Dinoflagellates, Academic Press, USA, https://doi.org/10.1201/9780203500217, 1996.
Tomas, C. R. (Ed.): Identifying Marine Phytoplankton, Academic Press, USA, ISBN 9780126930184, 1997.
Turra, A. and Denadai, M. R.: Linking biodiversity and global environmental changes in Brazilian coastal habitats, Braz. J. Oceanogr., 64, 3–4, https://doi.org/10.1590/S1679-87592016064sp2ed, 2016.
UNESCO: Chemical methods for use in marine environmental monitoring. Manual and Guides 12, Intergovernamental Oceanographic Commissiony, France, https://doi.org/10.25607/OBP-1419, 1983.
Vieillard, A. M., Newell, S. E., and Thrush, S. F.: Recovering from bias: A call for further study of underrepresented tropical and low-nutrient estuaries, J. Geophys. Res.-Biogeo., 125, e2020JG005766, https://doi.org/10.1029/2020JG005766, 2020.
Vieira, J. P., Garcia, A. M., and Grimm, A. M.: Evidences of El Niño effects on the mullet fishery of the Patos Lagoon Estuary, Braz. Arch. Biol. Techn., 51, 433–440, 2008.
Vieira, J. P., Garcia, A. M., and Moraes, L.: A assembleia de peixes, in: O Estuário da Lagoa dos Patos: Um Século de Transformações, edited by: Seeliger, U. and Odebrecht, C., FURG, Rio Grande, Brazil, 79–88, ISBN 978-85-7566-144-4, 2010.
Vieira, J. P., Garcia, A. M., and Lemos, V. M.: Species composition and abundance patterns of fish assemblages at shallow waters of Patos Lagoon estuary, GBIF [data set], https://doi.org/10.15468/kci8zb, 2020.
Vihervaara, P., D'Amato, D., Forsius, M., Angelstam, P., Baessler, C., Balvanera, P., Boldgiv, B., Bourgeron, P., Dick, J., Kanka, R., Klotz, S., Maass, M., Melecis, V., Petrík, P., Shibata, H., Tang, J., Thompson, J., and Zacharias, S.: Using long-term ecosystem service and biodiversity data to study the impacts and adaptation options in response to climate change: insights from the global ILTER sites network, Curr. Opin. Env. Sust., 5, 53–66, https://doi.org/10.1016/j.cosust.2012.11.002, 2013.
Von Ihering, H.: Rio Grande do Sul, Reuss, Weltpost-verlag P. Genschel, Germany, ISBN 0364521961, 9780364521960, 1885.
Weiss, G. (Ed.): Ictioplâncton Del Estuário De Lagoa Dos Patos, Brasil, Faculatad de Ciências Naturales y Museo, Universidad Nacional de La Plata, Argentina, https://doi.org/10.35537/10915/5041, 1981.
Welschmeyer, N. A.: Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments, Limnol. Oceanogr. Lett., 39, 1985–1992, https://doi.org/10.4319/lo.1994.39.8.1985, 1994.
Wilkinson, M. D., Dumontier, M., Aalbersberg, I. J., Appleton, G., Axton, M., Baak, A., Blomberg, N., Boiten, J.-W., da Silva Santos, L. B., Bourne, P. E., Bouwman, J., Brookes, A. J., Clark, T., Crosas, M., Dillo, I., Dumon, O., Edmunds, S., Evelo, C. T., Finkers, R., Gonzalez-Beltran, A., Gray, A. J. G., Groth, P., Goble, C., Grethe, J. S., Heringa, J., Hoen, P., Hooft, R., Kuhn, T., Kok, R., Kok, J., Lusher, S. J., Martone, M. E., Mons, A., Packer, A. L., Persson, B., Rocca-Serra, P., Roos, M., van Schaik, R., Sansone, S.-A., Schultes, E., Sengstag, T., Slater, T., Strawn, G., Swertz, M. A., Thompson, M., van der Lei, J., van Mulligen, E., Velterop, J., Waagmeester, A., Wittenburg, P., Wolstencroft, K., Zhao, J., and Mons, B.: The FAIR Guiding Principles for scientific data management and stewardship, Sci. Data., 3, 160018, https://doi.org/10.1038/sdata.2016.18, 2016.
WoRMS: Editorial Board, World Register of Marine Species, http://www.marinespecies.org at VLIZ (last access: 9 October 2021), 2021.