the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Global Acritarch Database ( > 110 000 occurrences)
Xiang Shu
Yong Lei
Daoliang Chu
Jacopo Dal Corso
Xiaokang Liu
Qin Ye
Hanchen Song
Lai Wei
Enhao Jia
Huyue Song
Wenchao Yu
Qingzhong Liang
Xinchuan Li
Yuyang Wu
Acritarchs are microfossils of unclear biological affinities, mostly considered to be algae, with great significance for studying the origin and evolution of early life on Earth. Acritarchs' data are currently dispersed across various research institutions and databases worldwide, lacking unified integration and standardization. Palynodata was the largest database of acritarchs, containing 14 fields, 111 295 entries, 812 061 metadata items, and 7369 references. However, it lacked references post-2007 and excluded geographic data. Here, we collect and organize previous data, adding 29 fields, 4531 entries, 2 238 366 metadata points, and 415 references, to build the Global Acritarch Database (GAD). The expanded database now contains a total of 43 fields, covering genera, species, and related geological information (geological timescale, location, modern latitude and longitude, paleolatitude and paleolongitude, stratum, and others), amounting to 115 860 entries, 3 050 852 metadata points, and 7791 references. Each entry is associated with fields that facilitate a better understanding of the geographical distribution and changes over geological timescales of acritarchs, thereby revealing their temporal and spatial distribution patterns and evolution throughout the history of the Earth. This article describes GAD version 1.0, which is available at https://doi.org/10.5281/zenodo.15208303 (Shu et al., 2025).
- Article
(4586 KB) - Full-text XML
- BibTeX
- EndNote
Acritarchs are organic-walled cysts of unicellular protists, first defined by Evitt (1963) as a group of “unknown and possibly varied biological affinities consisting of a central cavity enclosed by single or multiple layers of walls, mainly composed of organic materials” (Yin, 2018). Evitt (1963) also noted that acritarchs are an informal, practical classification category with no taxonomic ranks above the genus level, suggesting the use of the International Code of Botanical Nomenclature to name morphological genera and species without assigning them to a specific biological phylum (Wicander, 2002). Morphologically, acritarchs are typically single-celled microfossils ranging in size from a few micrometers to 1 mm. The most common shape is spherical, and they can be either smooth or covered with spines (Mendelson, 1987). Most of them have been interpreted as algal cysts (e.g., Colbath and Grenfell, 1995; Grey, 2005; Moczydłowska and Liu, 2022), while a few are related to non-algal origins (e.g., Butterfield, 2005; Schrank, 2003; Servais et al., 1997). For Precambrian acritarchs in particular, some specimens with dividing cells have been attributed to animal embryos/diapause cysts (Cohen et al., 2009; Xiao et al., 1998; Yin et al., 2007), giant sulfur bacteria (Bailey et al., 2007), or holozoan affinity (e.g., Huldtgren et al., 2011; Yin et al., 2020), which are important for understanding the origin and early evolution of animals. Following the foundational work of earlier researchers, Fensome et al. (1990) made significant advancements by compiling a comprehensive taxonomic index of acritarchs at the genus, species, and infraspecific levels, thereby significantly enhancing the standardization of classification criteria within the field. Acritarchs have been discovered in sedimentary rocks from marine and terrestrial aquatic environments, with records from all continents, spanning from the Proterozoic to the present. The oldest and most well-preserved acritarchs are derived from approximately 1.8 billion years ago in Mesoproterozoic (Buick, 2010), with evidence suggesting these rocks existed as far back as 2.5 billion years ago (Buick, 2010; Gaucher and Sprechmann, 2009). Acritarchs are valuable for determining chronological ages and biostratigraphic correlations for their high abundance, taxonomic diversity, and global distribution patterns (Lei et al., 2012), especially in Proterozoic and Paleozoic strata, where they are probably the only preserved fossils (Beraldi-Campesi, 2013; Wicander, 2002; Xiao and Narbonne, 2020). They are particularly valuable when combined with other fossil groups for regional and global paleobiogeography and paleoecology research (Dale, 2023; Lamb et al., 2009; Mudie et al., 2001). Additionally, acritarchs are primary producers at the base of the marine food chain in the Proterozoic and Paleozoic eras (Wicander, 2002) and played an important role in the evolution of global marine ecosystems (Falkowski and Knoll, 2011). Given their significance, it is crucial to establish a global database.
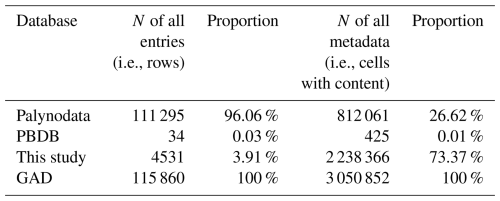
The compilation of acritarch databases dated back to the 1970s. Tappan and Loeblich (1973) pioneered systematic statistical work in this field by publishing a dataset covering the interval from 0–700 Ma. However, this early compilation exhibited relatively coarse temporal resolution and limited data. Even for the Ordovician, which had the highest data density, fewer than 500 species had been recorded. Between 1971 and 2010, John Williams compiled the “John Williams Index of Palaeopalynology”, which documented 1577 genera. A digitized version of this catalog is now archived in the Acritax online database (https://www.mikrotax.org/Acritax, last access: 21 April 2014). In the 1990s, with support from the Geological Survey of Canada (GSC), the Palynodata database was developed, integrating extensive acritarch records. Its final version, released in 2006, was published as GSC Open File 5793 (http://geopub.nrcan.gc.ca/moreinfo_e.php?id=225704, last access: 8 August 2008), containing 14 fields, 111 295 entries, 812 061 metadata items, and 7369 references.
Despite advances in acritarch research, several challenges remain. First, the morphological diversity and complex classification of acritarchs have limited our understanding of this group (Agić et al., 2015; Arouri et al., 1999; Bernard et al., 2015; Butterfield and Rainbird, 1998; Javaux and Marshal, 2006; Moldowan et al., 1996; Wang et al., 2022; Williams, 1998). Second, although many acritarchs have been discovered, global spatial and temporal distribution remains uneven, with certain regions experiencing relatively low amount of research (Gray and Boucot, 1989; Huntley et al., 2006; Jacobson, 1979; Lei et al., 2013; Schreck et al., 2017). Additionally, existing acritarch databases are often limited to specific regions or periods and lack comprehensive, systematic, and complete global coverage (Anderson et al., 2017; Bernardi et al., 2017; Chamberlain et al., 2016; Servais et al., 2003; Willman and Moczydłowska, 2011). These limitations hinder further research on acritarchs in geological history.
Here, we introduce a database that integrates global acritarch data from various geological periods, including the genus, geographical distribution, and geological timescales. In the following sections, we provide information regarding data sources and selection criteria; review and clean the definitions behind entries, fields, and metadata; and outline the process. We explore the extensive compiled spatial and temporal trends, discuss the future uses and limitations of the dataset, and address the ongoing goals of the database. By leveraging this global database, we can better understand the diversity and evolutionary patterns of acritarchs and reveal the structure and function of biological communities in geological history. It not only provides references for oil and gas exploration but also promotes interdisciplinary research. Through in-depth data mining and analysis, we can explore the acritarchs' stratigraphy and environmental and ecological issues throughout the Earth's history, ultimately providing new research ideas across different fields.
2.1 Compilation purpose
The affinities of acritarchs are primarily linked to algae, suggesting that acritarchs were the main contributors to primary productivity in early oceans, paving the way for the subsequent rise in consumer numbers (Agić, 2016; Daners et al., 2017). This implies that they played a crucial role in early marine environments and were important for maintaining ecological balance and carbon cycling. Quantitative analysis of fossils (e.g., acritarchs) from different strata allows for a better understanding of past changes in marine environments, including shifts in marine productivity, redox conditions, and carbon cycling. This aids in exploring the evolution of deep-time biological pumps and enhances our understanding of the processes and mechanisms behind the modern marine carbon cycle (Jia et al., 2022). Previous databases (Table 1), such as Palynodata (https://paleobotany.ru/palynodata, last access: 4 April 2025), containing a large number of acritarchs, exhibit several shortcomings: (1) the database only includes literature from 1842 to 2007, with no records for the following 17 years; (2) the numeric ages of strata in the database have not been updated; and (3) despite including 14 fields, Palynodata lacks critical information such as latitude, longitude, lithology, stratigraphy, and paleogeography. In contrast, the Paleobiology Database (PBDB, https://paleobiodb. org/, last access: 4 April 2025) only collects a small amount of acritarch data (866 entries in raw). In summary, previous databases exhibit issues such as incomplete data, difficulty in addressing fossil sampling biases, and inapplicability for studying spatiotemporal changes. Therefore, we aimed to build the Global Acritarch Database (GAD) to advance research in this field.
2.2 Metadata fields and criteria
GAD data come from PBDB, Palynodata, and published literature. From PBDB, 34 entries and 425 metadata points are sourced from 7 studies. The main component of the database was derived from Palynodata (Kroeck et al., 2022; Palynodata Inc. and White, 2008; Strother, 2008) contains 14 fields, 111 295 entries, and 812 061 metadata points, but it has not been updated since 2007, and its location information is limited to textual descriptions. In this study, we searched recent publications using Google Scholar using keywords (such as acritarchs, organic-walled microfossils) and collected 415 additional studies from 2008 to 2023. This collection includes 29 new fields, i.e., geological timescale (with uniform high-to-low levels: eon, era, period, epoch, and age), modern latitude and longitude, paleolatitude and paleolongitude, stratigraphy, and lithology, totaling 4531 entries and 2 238 366 metadata points. We have revised and updated the numeric age to the latest International Chronostratigraphic Chart (December 2024) (https://stratigraphy.org/, last access: 24 July 2025). Some of the entries that have not been updated include data without temporal information, entries spanning multiple periods, and ambiguously described Precambrian data. The aforementioned three sources together form a new database, GAD, containing 115 860 entries, 43 fields, and 3 050 852 metadata points. The database contains exclusively published data. The metadata primarily originated from original journal articles, supplements, or public repositories containing data tables. The included fields were organized to facilitate future updates of speciation/extinction models; taxonomic nomenclature corrections; data additions; and other research directions, such as genus and species information, lithological details, geological timescales, and sampling locations, thereby enabling continual data updates.
2.3 Data cleaning
To maintain clarity and consistency in data description, an “entry” refers to each genus and species along with its related metadata as reported in the literature (i.e., a row), while a “field” refers to the metadata collected for each entry (i.e., a column) (Judd et al., 2022).
To ensure accurate publishing and better utilization of the data, we have cleaned the data using the following steps.
-
Integration: All entries are integrated into a single data table, including entries that lack at least one type of information such as “genus name without species name”, “genus and species name without temporal information”, or “genus and species name without location information”. These were treated as separate entries to preserve them for possible future data replacements. Many Cambrian acritarch data were compiled by Palacios et al. (2009, 2012, 2014, 2017, 2020, 2021); Ordovician data by Le Hérissé et al. (2007, 2014, 2015, 2017), Paris et al. (2007), and Vecoli and Le Hérissé (2004); and Silurian and Devonian data by Vavrdová and Dašková (2011), Vavrdová and Svobodová (2010), and Vavrdová et al. (1996, 2011). Wherever possible, these compiled datasets were cross-checked with their original publications to ensure completeness, avoid errors, and fill in missing data or applicable fields.
-
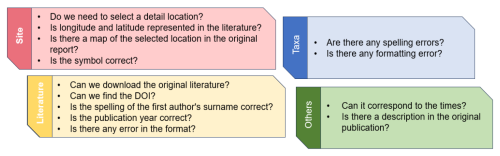
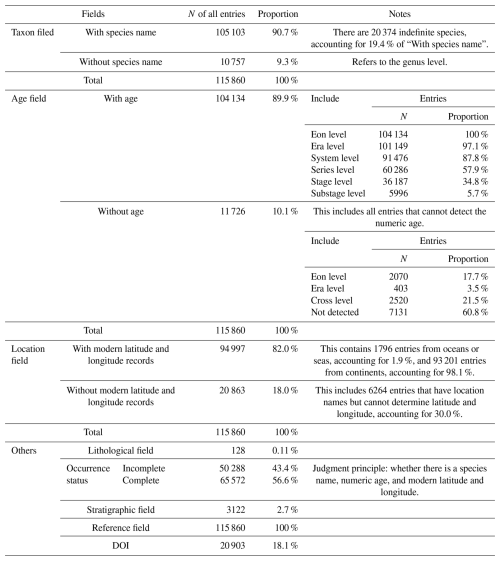
Taxonomic field: acritarchs are generally considered form-taxa and are morphologically identified at the genus/species level. During data cleaning, we regulated the representation of “sp.” and punctuation marks, such as question marks, commas, and parentheses, and minor spacing issues were removed to standardize the naming format and ensure proper characterization (Fig. 1). Considering that this database contains biological fossils, outdated taxonomies or misspellings may have led to analytical errors. We traced back to original publication to validate taxonomic reliability for each taxonomic entry (those questionable or illegitimate taxa, invalidly named taxa, taxa retained in open nomenclature, etc.) and implemented PyRate to check for spelling errors and inconsistencies among the listed species (Silvestro et al., 2014, 2019). The function check_names was utilized, which requires a text file with one species name per line. In the returned file, ranks 0 and 1 indicated the most likely spelling errors, whereas ranks 2 and 3 represented genuinely different names. It is noteworthy that this algorithm does not check for synonyms. Ultimately, species data accounted for 90.7 % of the database, with 19.4 % represented by “sp.”.
-
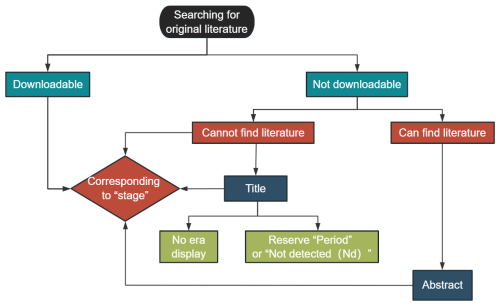
Age: includes 12 separate columns collectively. During data integration, several entries lacked temporal information or had insufficient resolution. Therefore, temporal information at the stage level was supplemented to ensure consistent information retrieval (Fig. 2). If precise data were unavailable, the highest possible resolution level was retained using the stage level as the primary reference, including numerical ages (in Ma) and period and stage information to provide relative ages. Ages were assigned by entering a numeric age and automatically matching to fill in relative age information, entering relative age information and automatically matching to fill in numeric age information or retaining manually entered numeric and relative ages. If the numeric age was not recorded in the literature, it was manually set the age of the top and bottom of its strata using the latest International Chronostratigraphic Chart (December 2024). In the absence of a precise numerical age, a stage position (i.e., early, middle, or late) was used to further define the relative age and match it with the numerical age. Entries with numerical age records accounted for 89.9 % of the database, and the remaining 11.1 % (11 726 entries) lacked numeric age data (Table 2). Additionally, entries with genus and species names were resolved to the stage level once supplemented, and they accounted for 34.8 % of the total data (excluding entries in which the numerical age could not be determined).
-
Location: includes nine separate columns collectively. During the data integration process, it has been observed that only broad location information was available. To enhance the application of the data in the geospatial field, a minor location information field was added, specifically the “point” (the center point of text location information) determined by latitude and longitude (Fig. 1). After supplementation, latitude and longitude information accounted for 82.0 % of the database. Modern latitude and longitude information were derived from detailed references to Google Maps (http://www.gditu.net, last access: 28 August 2023). If location information was not recorded in the literature, it was left blank. When it was impossible to determine the precise location, the latitude and longitude information of the center point of the broader location were added to the remarks field, affecting 5972 entries. Paleolatitude conversion primarily relied on GPlates (https://www.gplates.org/download/, last access: 8 October 2024, version 2.5), and map alignment was performed using QGIS (https://qgis.org/download/, last access: 8 October 2024, version 3.32.3). All maps were based on Scotese (2021).
-
Lithology and stratigraphy: this information covered only 0.11 % and 2.7 % of the total entries in the database, respectively, accounting for a very small proportion. The data on lithology and stratigraphy are the next priority for addition.
-
References: the reference field achieved 100 % coverage in the database. It included the main (first) author, publication year, and journal. DOIs of relevant literature were supplemented through Crossref (https://www.crossref.org/, last access: 3 June 2024). Concurrently, for the convenience of machine reading, special characters and garbled combinations in other applicable fields were deleted.
Each field was evaluated based on a set of standardized criteria to ensure consistency throughout the process (Fig. 1). Any issues discovered during this process were corrected. A summary of entries by fields is shown in Table 2.
3.1 Data statistics
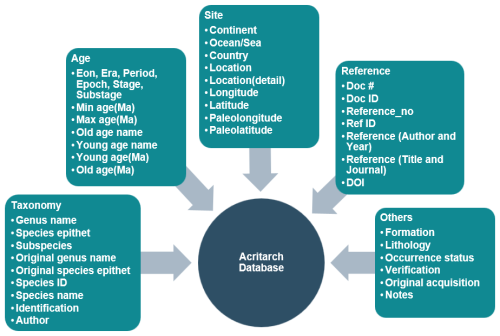
Each entry in the GAD is associated with a set of fields, all of which represent information related to fossils. There are 39 fields can be broadly divided into five categories (Fig. 3): (1) taxonomy, (2) age, (3) site, (4) reference, and (5) others. A basic description of these fields is summarized in Table 3, with details on how and why each field was assigned.
3.2 GAD statistics
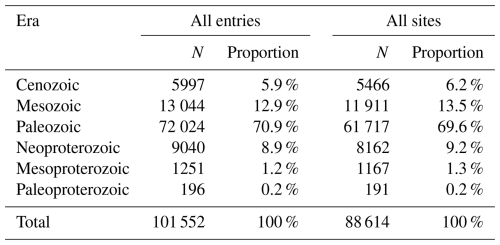
The GAD contains 115 860 entries from 7791 references, representing 1146 different sampling locations and records throughout geological history. Among these, 36 187 are marked as “stage level”, covering 101 out of the 102 stages in the Phanerozoic. In terms of biological fossil records, the database included 1456 genera and 9865 species (excluding those classified as sp.). During the process of correcting the numeric age, 7131 data points lacked a numeric age due to the inability to obtain geologic age from the original literature. The Paleozoic is the most well represented, accounting for 70.9 % of total entries (Table 4), followed by the Mesozoic (13 044 entries) and Neoproterozoic (9040 entries). Regarding the spatial distribution of acritarchs, 93 201 entries originated from the continent, with a small portion from oceanic or marine areas accounting for 1.9 %.
The sections below focus on fossil classification, literature sources, and paleogeographic and spatiotemporal distribution trends. These examples illustrate the unique aspects of this compilation method and demonstrate the potential of the database for promoting research in the paleoproductivity, paleoenvironment, and biological evolution.
3.3 Taxonomy statistics
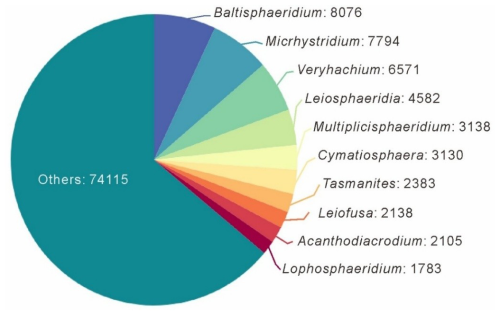
At the genus level, the database includes 1456 genera and 9865 species (excluding sp.). The top 10 genera, in terms of quantity that account for 36.0 % of the total data volume, are Baltisphaeridium (7.0 %), Micrhystridium (6.7 %), Veryhachium (5.7 %), Leiosphaeridia (3.9 %), Multiplicisphaeridium (2.7 %), Cymatiosphaera (2.7 %), Tasmanites (2.1 %), Leiofusa (1.8 %), Acanthodiacrodium (1.8 %), and Lophosphaeridium (1.5 %), and the specific number of entries can be obtained in Fig. 4. Baltisphaeridium (including 647 species accounting for 8076 entries in the database, including 337 entries having only the genus name and 1049 entries classified as sp.), the most abundant genus, has been present since the Precambrian period (approximately 1600 Ma) and is the most prolific during the Paleozoic Era.
3.4 Literature sources and statistics
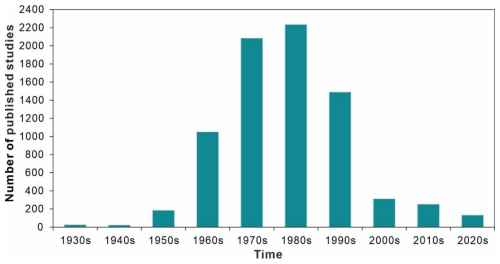
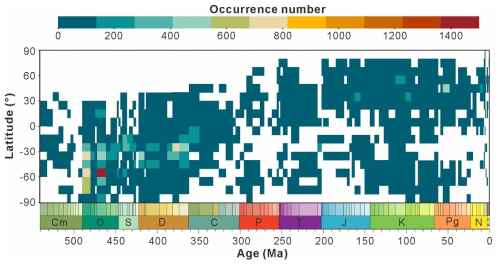
Data in this database were obtained from 7791 references spanning from 1842 to 2023. The temporal distribution of publication years is presented in Fig. 5. The average number of research outputs after 1930 (83.9 papers per year) is an order of magnitude greater than that before 1930 (0.12 papers per year). This difference is not significant in the number and was thus not displayed on the graph. Even the relatively lower research outputs of the 1950s and 2020s were more than 2.5-fold higher than the total output from the 1930s and 1940s combined over 20 years. More than half of research output occurred in the 1970s and 1980s, with 4320 papers accounting for 55.4 % of the total.
3.5 Temporal distribution
Figure 6a indicates that over a long timescale, data volume steadily increases during the Proterozoic but remains below 5000 entries, peaking in the Ediacaran with 3137 entries. However, there are almost no records for the Paleoproterozoic, accounting for only 1.9 % of the Proterozoic data. The Ordovician (Paleozoic) exhibits the highest number of entries at 21 880, followed by a decline to the Carboniferous low point of 1682 entries. Subsequently, a minor peak occurs during the Cretaceous (5959 entries) before the data volume drops below 5000 entries. Figure 6b presents the maximum data volume of 4431 entries during the Tremadocian (Ordovician), whereas the minimum is zero during the Jiangshanian (Cambrian). Two significant increases in data density occur at the intersections of Stage 10 and the Tremadocian (Cambrian–Ordovician) and between the Dapingian and Darriwilian (Ordovician). Four significant decreases occur at the transition between the Darriwilian and Floian (Ordovician), Darriwilian and Sandbian (Ordovician), Lochkovian and Pragian (Devonian), and Famennian and Tournaisian (Devonian–Carboniferous). Such data distribution may be attributed to (1) limited research intensity and (2) low temporal resolution in the study area, both of which constrain the availability of material for analysis.
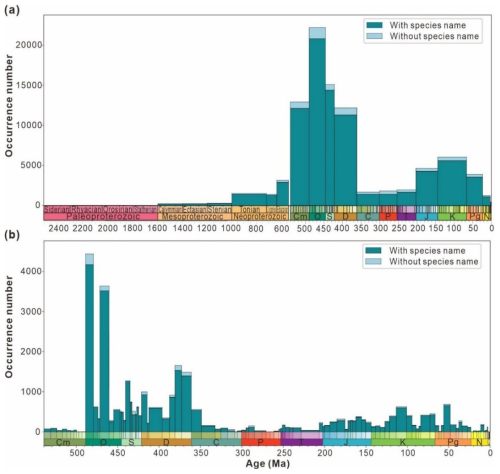
Figure 6The number of entries from the “have digital age” data split by “timescales include 2500 Ma” (a) and Phanerozoic (b) and binned by geologic stage. Each stage is divided into data with species name and data without species name for statistics according to the storage type of the genus and species field in the database.
3.6 Spatial distribution
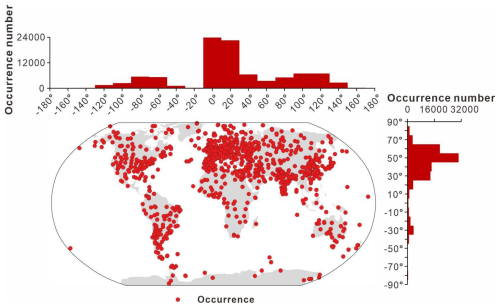
The spatial distribution of data collection is uneven. In terms of its modern distribution (Fig. 7), the peak in the longitudinal distribution lies primarily between −10 and 30°, with a small amount collected between −50 and −90° and 90 to 130°. According to the latitudinal distribution, most of the data are from the Northern Hemisphere (Europe, China, and North America) and predominantly between 25 and 65°, accounting for 82.0 % of the GAD. Figure 8 presents the modern geographic distribution by era. Most Precambrian data, primarily sourced from China and Europe, accounted for 86.4 % of the total, whereas most of the Phanerozoic data are from North America, Europe, Australia, and China, accounting for 93.2 %. The Cenozoic and Paleozoic data exhibit the widest spatial distribution (−176.2 to 176.1°), with the Paleozoic containing the highest quantity of data (61 717 entries, representing 69.6 % of the total geographic data).
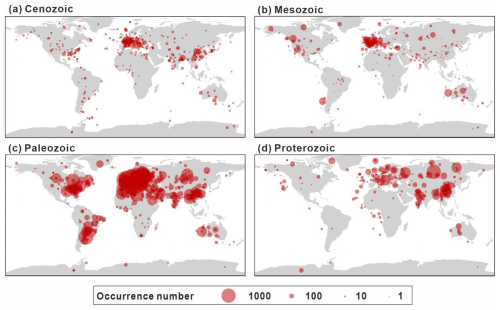
Figure 8Summary of the spatial distribution of sampling sites by era (a–d), with the size of each point scaled to the number of occurrences at each site. All panels are plotted on the same scale.
The paleogeographic distribution of data across periods (Fig. 9) highlights how data are concentrated in different regions over time. The diagram indicates that most of the data from the Cambrian to the Quaternary are from shallow marine environments, favoring continental edges. As the continents migrated northward from the Mesozoic to the Cenozoic, records begin to concentrate in the mid-latitude regions in the Northern Hemisphere. Taking the peak values of each period as examples and starting with the Cambrian, the highest data concentration is observed between −35 and −45° (3688 entries), mainly in Gondwana and the Baltic, which shifted to −25 and −35° (3708 entries) by the Ordovician. In the Carboniferous, the highest data concentration is near −5 to −15° (468 entries) in the North American and Eurasian plates. In the Permian, data are evenly distributed across the mid-latitude regions near the coast of the Tethys Ocean in both hemispheres. Thereafter, fossil records start to tilt towards the mid-latitude regions of the Northern Hemisphere (such as North America, Europe, and Asia) during the Mesozoic and Cenozoic. The highest data concentrations were between 25 and 35° during the Triassic and moved to between 35 and 45° and between 45 and 55° during the Jurassic–Cretaceous and Paleogene–Quaternary periods, respectively.
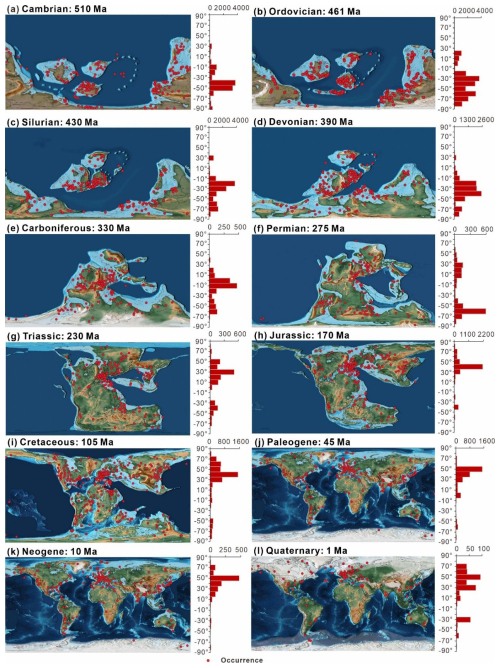
Figure 9Summary of the paleogeographic spatial distribution of sampling sites (a–l) separated by geologic period. Histograms to the right of each map show the relative latitudinal distribution of all unique sampling sites within 10° bins, with the horizontal axis representing the number of occurrences. The chronology number indicates the exact point in time for the map selection. For example, Ordovician: 461 Ma represents the Middle Ordovician. All maps are based on Scotese (2021).
Table 3Detailed description and notes for each field.
Incidentally, “Nd” represents “not detected”; it is just that the corresponding information cannot be obtained from the original literature.
3.7 Spatial–temporal trends in proxy values
The large volume and consistent structure of data in GAD provide opportunities to investigate research trends in acritarchs (e.g., regional research focus and taxonomic variations). Figure 10 presents heatmaps for each time interval from database entries, where the data is temporally averaged by stage level and spatially into 10° paleolatitude bins. Vertical trends indicate the latitudinal gradient for any given “stage”, while horizontal trends indicate the temporal evolution of entries within latitudinal intervals. Notably, the data volume is predominantly observed in the mid- to low latitudes of the Southern Hemisphere during the Paleozoic, with over 400 entries and peaks reaching above 1400. A clear migration pattern is observed as the majority of data shift from the Southern Hemisphere to the Northern Hemisphere over time. Tectonic movements appear to have been a significant contributing factor since the formation of Pangaea about 250 million years ago, as Gondwana gradually split apart. The plates of South America, Africa, Antarctica, Australia, and India have been drifting northward progressively, affecting the geographical pattern and biodiversity of the Earth (Park, 1988). However, the spatial–temporal trend may be influenced by sampling biases arising from uneven research distribution as well as inherent taxonomic uncertainties associated with acritarchs. The heatmap (Fig. 10) clearly indicates that all entries exhibited discontinuous spatial and temporal coverage, but the Mesozoic (Cretaceous) and Paleozoic (Ordovician and Devonian) generally exhibited good coverage, extending from 30 to −90°. During the mid-Cretaceous, coverage reached 90 %. In contrast, the Paleozoic (middle to late Cambrian and Permian), Mesozoic (Jurassic), and Cenozoic exhibited highly discontinuous geographic coverage with a significantly reduced range.
All data for GAD (version 1.0) can be found on Zenodo: https://doi.org/10.5281/zenodo.15208303 (Shu et al., 2025). A static copy of GAD (version 1.0) is archived in the Geobiology database (https://geobiologydata.cug.edu.cn/, GeobiologyData, 2025). We will continuously update and enhance the database and welcome collaboration with existing compilation authors to expand its content.
All available example codes and auxiliary functions have been uploaded on Zenodo: https://doi.org/10.5281/zenodo.15147118 (Shu, 2025).
The Global Acritarch Database (GAD) is a global acritarch database that integrates data from Palynodata and Paleobiology Database (PBDB) and additional published literature not included in previous collections. Building on the foundation of Palynodata, which originally contained 14 fields, 111 295 entries, 812 061 metadata points, and 7369 references, GAD added 29 new fields, 4531 new entries, 2 238 366 new metadata points, and 415 new references, resulting in a database comprising 115 860 entries, 43 fields, 3 050 852 metadata points, and 7791 references. GAD represents records from 1146 different sampling sites spanning geological history from the Precambrian to the Phanerozoic. The fossil records include 1456 genera and 9865 species (excluding sp.). Additionally, the database records information related to occurrences, such as stratigraphy, lithology, and paleogeography. Among all entries, Paleozoic data are the most abundant, accounting for 70.9 % of the total, followed by 13 044 Mesozoic, 9040 Neoproterozoic, 5997 Cenozoic, 1251 Mesoproterozoic, and 196 Paleoproterozoic entries. Regarding the spatial distribution of acritarchs, 93 201 are derived from continents and primarily concentrated in Europe, North America, China, and India, with the remaining 1.9 % originating from oceanic or marine regions.
Although substantial efforts have been made, the dataset remains incomplete. For example, information regarding the size dimensions of acritarchs, lithology, and strata are lacking and will be continuously supplemented in the future. Additionally, while meticulous care was taken to ensure accuracy, some errors may have been overlooked due to the sheer volume of data. When reusing GAD, we recommend citing both the GAD and original data sources to ensure proper attribution. Any issues or omissions discovered by the end users can be reported to us, and the relevant information will be updated in future versions of the database. GAD is expected to remain a valuable resource for ongoing and future research.
XS: collected data, conducted database statistical analysis, and drafted the manuscript; HS, DC, YW, XiaL, EJ, YF, YD, WY, HaS: they have done a lot of work in expanding and adjusting metadata structures, fields, and other information during the data collection process; HuS: technical guidance on ancient and modern geographic maps; LW, XiaL, QL, XinL, HY: technical support for computer language writing, literature collection, semi-automatic data extraction, and data cleaning and screening; HS, YL, JDC, QY, YW, XiaL, EJ: provided valuable revision suggestions for the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We are deeply grateful to Jan Hennissen and an anonymous reviewer for their meticulous peer review and invaluable suggestions, which have greatly enhanced the quality of this manuscript. We also would like to express our gratitude to the researchers at Palynodata and Paleobiology Database for collecting and organizing metadata.
This study was supported by the National Natural Science Foundation of China (grant no. 42325202); the National Key Research and Development Program (grant nos. 2023YFF0804000 and 2024YFF0810300), project B08030 of 111; and the Natural Science Foundation of Hubei Province (grant no. 2023AFA006), the project of Fundamental Research Funds for National Universities, China University of Geosciences (Wuhan) (grant no. 2024XLB82); as well as the Fundamental Research Program of Shanxi Province (grant no. 20210302123378).
This paper was edited by Frédéric Gazeau and reviewed by Jan Hennissen and one anonymous referee.
Agić, H.: Fossil focus: Acritarchs, Palaeontology, 6, 1–13, 2016.
Agić, H., Moczydłowska, M., and Yin, L. M.: Affinity, life cycle, and intracellular complexity of organic-walled microfossils from the Mesoproterozoic of Shanxi, China, J. Paleontol., 89, 28–50, https://doi.org/10.1017/jpa.2014.4, 2015.
Anderson, R. P., Macdonald, F. A., Jones, D. S., McMahon, S., and Briggs, D. E. G.: Doushantuo-type microfossils from latest Ediacaran phosphorites of northern Mongolia, Geology, 45, 1079–1082, https://doi.org/10.1130/G39576.1, 2017.
Arouri, K., Greenwood, P. F., and Walter, M. R.: A possible chlorophycean affinity of some Neoproterozoic acritarchs, Org. Geochem., 30, 1323–1337, https://doi.org/10.1016/s0146-6380(99)00105-9, 1999.
Bailey, J. V., Joye, S. B., Kalanetra, K. M., Flood, B., and Corsetti, F. A.: Evidence of giant Sulphur bacteria in Neoproterozoic phosphorites, Nature, 445, 198–201, https://doi.org/10.1038/nature05457, 2007.
Beraldi-Campesi, H.: Early life on land and the first terrestrial ecosystems, Ecol. Process., 2, 1, https://doi.org/10.1186/2192-1709-2-1, 2013.
Bernard, S., Benzerara, K., Beyssac, O., Balan, E., and Brown Jr., G. E.: Evolution of the macromolecular structure of sporopollenin during thermal degradation, Heliyon, 1, e00034, https://doi.org/10.1016/j.heliyon.2015.e00034, 2015.
Bernardi, M., Petti, F. M., Kustatscher, E., Franz M., Hartkopf-Fröder, C., Labandeira, C. C., Wappler, T., van Konijnenburg-van Cittert, J. H., Peecook, B. R., and Angielczyk, K. D.: Late Permian (Lopingian) terrestrial ecosystems: A global comparison with new data from the low-latitude Bletterbach Biota, Earth-Sci. Rev., 175, 18–43, https://doi.org/10.1016/j.earscirev.2017.10.002, 2017.
Buick, R.: Ancient acritarchs, Nature, 463, 885–886, https://doi.org/10.1038/463885a, 2010.
Butterfield, N. J.: Probable Proterozoic fungi, Paleobiology, 31, 165–182, https://doi.org/10.1666/0094-8373(2005)031<0165:PPF>2.0.CO;2, 2005.
Butterfield, N. J. and Rainbird, R. H.: Diverse organic-walled fossils, including “possible dinoflagellates,” from the early Neoproterozoic of arctic Canada, Geology, 26, 963–966, https://doi.org/10.1130/0091-7613(1998)026<0963:DOWFIP>2.3.CO;2, 1998.
Chamberlain, J., Chamberlain, R., and Brown, J. A.: Mineralized alga and acritarch dominated microbiota from the Tully Formation (Givetian) of Pennsylvania, USA, Geosciences, 6, 57, https://doi.org/10.3390/geosciences6040057, 2016.
Cohen, P. A., Knoll, A. H. and Kodner, R. B.: Large spinose microfossils in Ediacaran rocks as resting stages of early animals, P. Natl. Acad. Sci. USA, 106, 6519–6524, https://doi.org/10.1073/pnas.0902322106, 2009.
Colbath, G. K. and Grenfell, H. R.: Review of biological affinities of Paleozoic acid-resistant, organic-walled eukaryotic algal microfossils (including “acritarchs”), Rev. Palaeobot. Palynol., 86, 287–314, https://doi.org/10.1016/0034-6667(94)00148-d, 1995.
Dale, B.: Paleontological evidence for dinoflagellates and ciliates as early eukaryotes, J. Mar. Sci. Eng., 11, 533, https://doi.org/10.3390/jmse11030533, 2023.
Daners, G., Herisse, A. L., Breuer, P., and Veroslavsky, G.: Pragian–Emsian palynomorphs from the Cordobés Formation, Norte Basin, Uruguay: Stratigraphically restricted and regionally correlative palynological events in the cool-water Malvinokaffric Realm, Palynology, 41, 121–137, https://doi.org/10.1080/01916122.2017.1366115, 2017.
Evitt, W. R.: A discussion and proposals concerning fossil dinoflagellates, hystrichospheres, and acritarchs, I., P. Natl. Acad. Sci. USA, 49, 158–164, https://doi.org/10.1073/pnas.49.2.158, 1963.
Falkowski, P. and Knoll, A. H. (Eds.): Evolution of primary producers in the sea, Academic Press, https://doi.org/10.5860/choice.45-3183, 2011.
Fensome, R. A., Williams, G. L., Barss, M. S., Freeman, J. M. and Hill, J. M.: Acritarchs and fossil prasinophytes: an index to genera, species and infraspecific taxa, AASP Contrib. Ser., 25, 1–771, 1990.
Gaucher, C. and Sprechmann, P.: Neoproterozoic acritarch evolution, Dev. Precamb. Geol., 16, 319–326, https://doi.org/10.1016/S0166-2635(09)01622-3, 2009.
GeobiologyData: https://geobiologydata.cug.edu.cn/ (last access: 22 April 2025), 2025.
Gray, J. and Boucot, A. J.: Is Moyeria a euglenoid?, Lethaia, 22, 447–456, https://doi.org/10.1111/j.1502-3931.1989.tb01449.x, 1989.
Grey, K.: Ediacaran palynology of Australia, Memoir of the Association of Australasian Palaeontologists, 31, 1–439, 2005.
Huldtgren, T., Cunningham, J., Yin, C., Stampanoni, M., Marone, F., Donoghue, P. C. J., and Bengtson, S.: Fossilized nuclei and germination structures identify Ediacaran “animal embryos” as encysting protists, Science, 334, 1696–1699, https://doi.org/10.1126/science.1209537, 2011.
Huntley, J., Xiao, S., and Kowalewski, M.: 1.3 billion years of acritarch history: An empirical morphospace approach, Precamb. Res., 144, 52–68, https://doi.org/10.1016/j.precamres.2005.11.003, 2006.
Jacobson, S. R.: Acritarchs as paleoenvironmental indicators in Middle and Upper Ordovician rocks from Kentucky, Ohio and New York, J. Paleontol., 53, 1197–1212, 1979.
Javaux, E. J. and Marshal, C. P.: A new approach in deciphering early protist paleobiology and evolution: Combined microscopy and microchemistry of single Proterozoic acritarchs, Rev. Palaeobot. Palynol., 139, 1–15, https://doi.org/10.1016/j.revpalbo.2006.01.005, 2006.
Jia, E., Song, H., Lei, Y., Luo, G., and Jiang, S.: Paleozoic-Mesozoic turnover of marine biological pump and Mesozoic plankton revolution, Chin. Sci. Bull., 67, 1660–1676, https://doi.org/10.1360/TB-2021-1220, 2022.
Judd, E. J., Tierney, J. E., Huber, B. T., Wing, S. L., Lunt, D. J., Ford, H. L., Inglis, G. N., McClymont, E. L., O'Brien, C. L., Rattanasriampaipong, R., Si, W., Staitis, M. L., Thirumalai, K., Anagnostou, E., Cramwinckel, M. J., Dawson, R. R., Evans, D., Gray, W. R., Grossman, E. L., Henehan, M. J., Hupp, B. N., MacLeod, K. G., O'Connor, L. K., Sanchez Montes, M. L., Song, H., and Zhang, Y. G.: The PhanSST global database of Phanerozoic sea surface temperature proxy data, Sci. Data, 9, 753, https://doi.org/10.1038/s41597-022-01826-0, 2022.
Kroeck, D. M., Mullins, G., Zacaï, A., Monnet, C., and Servais, T.: A review of Paleozoic phytoplankton biodiversity: Driver for major evolutionary events?, Earth-Sci. Rev., 232, 104113, https://doi.org/10.1016/j.earscirev.2022.104113, 2022.
Lamb, D. M., Awramik, S. M., Chapman, D. J., and Zhu, S.: Evidence for eukaryotic diversification in the ∼1800 million-year-old Changzhougou Formation, North China, Precamb. Res., 173, 93–104, https://doi.org/10.1016/j.precamres.2009.05.005, 2009.
Le Hérissé, A., Al-Ruwaili, M., Miller, M., and Vecoli, M.: Environmental changes reflected by palynomorphs in the early Middle Ordovician Hanadir Member of the Qasim Formation, Saudi Arabia, Revue de micropaléontologie, 50, 3–16, https://doi.org/10.1016/j.revmic.2007.01.010, 2007.
Le Hérissé, A., Steemans, P., Wellman, C., and Vecoli, M.: Darriwilian (Middle-Ordovician) elements of primitive vegetation, marine palynomorphs and problematic microfossils, from the Saq/Qasim transitional beds in core QSIM 801, central Saudi Arabia. Discussion with eustatic and climatic events, in: 9th European Palaeobotany-Palynology conference, 26–31 August 2014, Padova, Italy, https://www.academia.edu/107773090 (last access: 25 July 2025), 2014.
Le Hérissé, A., Molyneux, S. G., and Miller, M. A.: Late Ordovician to early Silurian acritarchs from the Qusaiba-1 shallow core hole, central Saudi Arabia, Rev. Palaeobot. Palynol., 212, 22–59, https://doi.org/10.1016/j.revpalbo.2014.08.016, 2015.
Le Hérissé, A., Vecoli, M., Guidat, C., Not, F., Breuer, P., Wellman, C., and Steemans, P.: Middle Ordovician acritarchs and problematic organic-walled microfossils from the Saq-Hanadir transitional beds in the QSIM-801 well, Saudi Arabia, Rev. Micropaléontologie, 60, 289–318, https://doi.org/10.1016/j.revmic.2017.08.001, 2017.
Lei, Y., Servais, T., Feng, Q., and He, W.: The spatial (nearshore–offshore) distribution of latest Permian phytoplankton from the Yangtze Block, South China, Palaeogeogr. Palaeocl. Palaeoecol., 363–364, 151–162, https://doi.org/10.1016/j.palaeo.2012.09.010, 2012.
Lei, Y., Servais, T., and Feng, Q.: The diversity of the Permian phytoplankton, Rev. Palaeobot. Palynol., 198, 145–161, https://doi.org/10.1016/j.revpalbo.2013.03.004, 2013.
Mendelson, C. V.: Acritarchs. Series in Geology, Notes Short Course, 18, 62–86, https://doi.org/10.1017/S0271164800001500, 1987.
Moczydłowska, M. and Liu, P.: Ediacaran algal cysts from the Doushantuo Formation, South China, Geol. Mag., 159, 1050–1070, https://doi.org/10.1017/S0016756820001405, 2022.
Moldowan, J. M., Dahl, J., Jacobson, S. R., Huizinga, B. J., Fago, F. J., Shetty, R., Watt, D. S., and Peters, K. E.: Chemostratigraphic reconstruction of biofacies: Molecular evidence linking cyst-forming dinoflagellates with pre-Triassic ancestors, Geology, 24, 159–162, https://doi.org/10.1130/0091-7613(1996)024<0159:CROBME>2.3.CO;2, 1996.
Mudie, P. J., Aksu, A. E., and Yasar, D.: Late Quaternary dinoagellate cysts from the Black, Marmara and Aegean seas: Variations in assemblages, morphology and paleosalinity, Mar. Micropaleontol., 43, 155–178, https://doi.org/10.1016/S0377-8398(01)00006-8, 2001.
Palacios, T., Jensen, S., Barr, S. M., and White, C. E.: Acritarchs from the MacLean Brook Formation, southeastern Cape Breton Island, Nova Scotia, Canada: New data on Middle Cambrian–Lower Furongian acritarch zonation, Palaeogeogr. Palaeocl. Palaeoecol., 273, 123–141, https://doi.org/10.1016/j.palaeo.2008.12.006, 2009.
Palacios, T., Jensen, S., White, C. E., and Barr, S. M.: Cambrian acritarchs from the Bourinot belt, Cape Breton Island, Nova Scotia: Age and stratigraphic implications, Can. J. Earth Sci., 49, 289–307, https://doi.org/10.1139/e11-010, 2012.
Palacios, T., Jensen, S., Sánchez, I. C., and Mus, M. M.: First Lower Cambrian record of Wiwaxia from north- west Gondwana: Small carbonaceous fossils from the Lancara Formation, Cantabrian Mountains, northern Spain. Programme, Abstracts and AGM Papers, in: 58th Annual Meeting of the Palaeontological Association, University of Leeds, Leeds, UK, https://www.academia.edu/15114883 (last access: 24 July 2025), 2014.
Palacios, T., Jensen, S., Barr, S. M., White, C. E., and Miller, R. F.: Acritarchs from the Hanford Brook Formation, New Brunswick, Canada: New biochronological constraints on the Protolenus elegans Zone and the Cambrian Series 2–3 transition, Geol. Mag., 154, 571–590, https://doi.org/10.1017/S0016756816000224, 2017.
Palacios, T., Högström, A. E., Ebbestad, J. O. R., Agić, H., Høyberget, M., Jensen, S., Meinhold, G., and Taylor, W. L.: Acritarchs from the Duolbagáisá Formation (Cambrian Series 2, Miaolingian) on the Digermulen Peninsula, Finnmark, Arctic Norway: Towards a high-resolution Cambrian chronostratigraphy, Geol. Mag., 157, 2051–2066, 2020.
Palacios, T., Jensen, S., Álvaro, J. J., Zaldeugui, J. S., Eguiluz, L., Corfu, F., and Ibarguchi, J. G.: Acritarch-based chronostratigraphic and radiometric calibration of the Cambrian volcanosedimentary Vallehondo and Playón formations in the Cambrian Ossa-Morena Rift, Spain, Palaeogeogr. Palaeoclim. Palaeoecol., 565, 110216, https://doi.org/10.1016/j.palaeo.2021.110216, 2021.
Palynodata Inc. and White, J. M.: Palynodata Datafile: 2006 version, with Introduction by J. M. White, Geological Survey of Canada Open File 5793, 1 CD-ROM, Geological Survey of Canada, https://doi.org/10.4095/225704, 2008.
Paris, F., Hérissé, A. L., Monod, O., Kozlu, H., Ghienne, J. F., Dean, W. T., Vecoli, M., and Günay, Y.: Ordovician chitinozoans and acritarchs from southern and southeastern Turkey Revue de micropaléontologie, 50, 81–107, https://doi.org/10.1016/j.revmic.2006.11.004, 2007.
Park, R. G.: Geological structures and moving plates, Springer Science & Business Media, https://doi.org/10.1007/978-94-017-1685-7, 1988.
Schrank, E.: Small acritarchs from the Upper Cretaceous: Taxonomy, biological affinities and palaeoecology, Rev. Palaeobot. Palynol., 123, 199–235, https://doi.org/10.1016/S0034-6667(02)00228-2, 2003.
Schreck, M., Nam, S. I., Clotten, C., Fahl, K., De Schepper, S., Forwick, M., and Matthiessen, J.: Neogene dinoflagellate cysts and acritarchs from the high northern latitudes and their relation to sea surface temperature, Mar. Micropaleontol., 136, 51–65, https://doi.org/10.1016/j.marmicro.2017.09.003, 2017.
Scotese, C. R.: An atlas of Phanerozoic paleogeographic maps: The seas come in and the seas go out, Annu. Rev. Earth Planet. Sci., 49, 679–728, https://doi.org/10.1146/annurev-earth-081320-064052, 2021.
Servais, T., Brocke, R., Fatka, O., Herisse, A. L., and Molyneux, S. G.: Value and meaning of the term acritarch, in: Acritarcha in Praha 1996, Acta Universitatis Carolinae, edited by: Fatka, O. and Servais, T., Geologica, 40, 631–643, 1997.
Servais, T., Li, J., Molyneux, S., and Raevskaya, E.: Ordovician organic-walled microphytoplankton (acritarch) distribution: The global scenario, Palaeogeogr. Palaeoclim. Palaeoecol., 195, 149–172, https://doi.org/10.1016/S0031-0182(03)00306-7, 2003.
Shu, X.: Code encountered during the drawing process, Zenodo [code], https://doi.org/10.5281/zenodo.15147118, 2025.
Shu, X., Song, H., Lei, Y., Chu, D., Dal Corso, J., Liu, X., Song, H., Wei, L., Jia, E., Feng, Y., Du, Y., Song, H., Yu, W., Liang, Q., Li, X., Yao, H., and Wu, Y.: Global Acritarch Database, Zenodo [data set] https://doi.org/10.5281/zenodo.15208303, 2025.
Silvestro, D., Salamin, N., and Schnitzler, J.: PyRate: A new program to estimate speciation and extinction rates from incomplete fossil data, Meth. Ecol. Evol., 5, 1126–1131, https://doi.org/10.1111/2041-210X.12263, 2014.
Silvestro, D., Salamin, N., Antonelli, A., and Meyer, X.: Improved estimation of macroevolutionary rates from fossil data using a Bayesian framework, Paleobiology, 45, 546–570, https://doi.org/10.1017/pab.2019.23, 2019.
Strother, P. K.: A speculative review of factors controlling the evolution of phytoplankton during Paleozoic time, Revue de Micropaleontology, 51, 9–21, https://doi.org/10.1016/j.revmic.2007.01.007, 2008.
Tappan, H. and Loeblich Jr., A. R.: Evolution of the oceanic plankton, Earth-Sci. Rev., 9, 207–240, https://doi.org/10.1016/0012-8252(73)90092-5, 1973.
Vavrdová, M. and Dašková, J.: Middle Devonian palynomorphs from southern Moravia: An evidence of rapid change from terrestrial deltaic plain to carbonate platform conditions, Geol. Carpathica, 62, 109–119, https://doi.org/10.2478/v10096-011-0010-2, 2011.
Vavrdová, M. and Svobodová, M.: Amphitheca isaacsonii gen. et sp. nov. (Acritarcha) from the Ananea Formation (Silurian/Devonian transition), southern Peru, Journal of the National Museum (Prague), Natural History Series, 179, 189–196, 2010.
Vavrdová, M., Bek, J., Dufka, P., and Isaacson, P. E.: Palynology of the Devonian (Lochkovian to tournaisian) sequence, Madre de Dios Basin, northern Bolivia, Vestnik Ceskeho geologickeho ustavu, 71, 333–349, 1996.
Vavrdová, M., Isaacson, P. E., and Díaz-Martínez, E.: Early Silurian-Early Devonian acritarchs and prasinophytes from the Ananea and San Gabán Formations, southern Peru and their paleogeographic implications, Revista Española de Micropaleontología, 43, 157–172, 2011.
Vecoli, M. and Le Hérissé, A.: Biostratigraphy, taxonomic diversity and patterns of morphological evolution of Ordovician acritarchs (organic-walled microphytoplankton) from the northern Gondwana margin in relation to palaeoclimatic and palaeogeographic changes, Earth-Sci. Rev., 67, 267–311, https://doi.org/10.1016/j.earscirev.2004.03.002, 2004.
Wang, K., Xu, H. H., and Yin, L. M.: A palynological assemblage from the Cambrian (Series 2, Stage 4) of Shandong Province, China, and its implications to the transition from algae to land plants, Rev. Palaeobot. Palynol., 301, 104645, https://doi.org/10.1016/j.revpalbo.2022.104645, 2022.
Wicander, R.: Acritarchs: Proterozoic and Paleozoic enigmatic organic-walled microfossils, edited by: Hoover, R. B. and Levin, G. V., Instruments, Methods, and Mission for Astrobiology IV. Proc. Soc. Phot.-Opt. Instrum. Eng. (SPIE), 4495, 331–340, https://doi.org/10.1117/12.454771, 2002.
Williams, G. L.: Dinoflagellates, acritarchs and tasmanitids, in: Introduction to Marine Micropaleontology, Elsevier, 293–326, https://doi.org/10.1016/B978-044482672-5/50013-1, 1998.
Willman, S. and Moczydłowska, M.: Acritarchs in the Ediacaran of Australia – Local or global significance? Evidence from the Lake Maurice West 1 drillcore, Rev. Palaeobot. Palynol., 166, 12–28, https://doi.org/10.1016/j.revpalbo.2011.04.005, 2011.
Xiao, S. and Narbonne, G. M.: The Ediacaran Period, in: Geologic Time Scale 2020 (Volume 1), edited by: Gradstein, F. M., Ogg, J. G., Schmitz, M. D., and Ogg, G. M., Elsevier, Oxford, 521–561, https://doi.org/10.1016/B978-0-12-824360-2.00018-8, 2020.
Xiao, S., Zhang, Y., and Knoll, A. H.: Three-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite, Nature, 391, 553–558, https://doi.org/10.1038/35318, 1998.
Yin, L.: Illustrated book of organic-walled microfossils, Zhejiang University Press, Zhejiang, ISBN 978-7-308-18633-9, 2018.
Yin, L., Zhu, M., Knoll, A. H., Yuan, X., Zhang, J., and Hu, J.: Doushantuo embryos preserved inside diapause egg cysts, Nature, 446, 661–663, https://doi.org/10.1038/nature05682, 2007.
Yin, Z., Sun, W., Liu, P., Zhu, M., and Donoghue, P. C. J.: Developmental biology of Helicoforamina reveals holozoan affinity, cryptic diversity, and adaptation to heterogenous environments in the early Ediacaran Weng'an biota (Doushantuo Formation, South China), Sci. Adv., 6, eabb0083, https://doi.org/10.1126/sciadv.abb0083, 2020.