the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Global database of actual nitrogen loss rates in coastal and marine sediments
Yongkai Chang
Ehui Tan
Dengzhou Gao
Cheng Liu
Zongxiao Zhang
Zhixiong Huang
Jianan Liu
Yu Han
Zifu Xu
Bin Chen
Shuh-Ji Kao
Denitrification and anaerobic ammonium oxidation (anammox) convert reactive nitrogen to inert N2 and play vital roles in nitrogen removal in coastal and marine ecosystems, weakening the adverse effects caused by terrestrial excessive nitrogen inputs. Given the importance of denitrification and anammox in the nitrogen cycle, several studies have measured denitrification and anammox through intact core incubations across different systems, and nitrogen loss processes are affected by a series of environmental factors such as organic carbon, nitrate, dissolved oxygen and temperature. However, a global synthesis of actual nitrogen loss rates is lacking, and how environmental factors regulate nitrogen loss remains unclear. Therefore, we have compiled a database of nitrogen loss rates, including denitrification and anammox in coastal and marine systems, from the published literature. This database includes 473, 466 and 255 measurements for total nitrogen loss, denitrification and anammox, respectively. This work deepens our understanding of the spatial and temporal distribution of denitrification, anammox and the relative contribution of anammox to total nitrogen loss, and their corresponding environmental controls. To our knowledge, the constructed database offers for the first time a comprehensive overview of actual nitrogen loss rates in coastal and marine ecosystems on a global scale. This database can be utilized to compare nitrogen loss rates of different regions, identify the key factors regulating these rates and parameterize biogeochemical models in the future. This database is available from the Figshare repository at https://doi.org/10.6084/m9.figshare.27745770.v3 (Chang et al., 2024).
- Article
(5475 KB) - Full-text XML
- BibTeX
- EndNote
The production of anthropogenic reactive nitrogen has intensified remarkably since the mid-20th century to meet the increasing global population (Kennedy, 2021). It is estimated that nitrogen is entering Earth's ecosystems at more than twice its natural rate, drastically disrupting the pristine nitrogen cycle (Canfield et al., 2010). Much of the excess nitrogen, primarily in the form of nitrate, is conveyed downriver to coastal and marine systems due to the low use efficiency of crops (Cui et al., 2013), resulting in a series of environmental issues including harmful algal blooms, eutrophication and hypoxia (Dai et al., 2023). Consequently, it is critical to understand the transformations, particularly the fates of reactive nitrogen, considering the fact that the nitrogen cycle has been intensively altered and is currently functioning beyond the safe operating space for humanity (Richardson et al., 2023).
Denitrification and anammox (Anaerobic Ammonium Oxidation) are two key nitrogen loss processes in aquatic environments, playing important roles in mitigating the adverse effects of excessive nitrogen inputs (Chen et al., 2021; Tan et al., 2022). Denitrification is the sequential reduction of nitrate, nitrite, nitric oxide and nitrous oxide (N2O) to dinitrogen gas (N2), which is the most energetically favourable respiratory pathway in the absence of oxygen (Devol, 2015), serving as the predominant mechanism for nitrogen loss in coastal ecosystems (Damashek and Francis, 2018; Deng et al., 2024). Anaerobic ammonium oxidation (Anammox), an alternate nitrogen loss pathway, utilizes nitrite and ammonium to generate N2 with no greenhouse gas N2O production under anaerobic conditions (van de Graaf et al., 1995) and is a chemoautotrophic process with no direct demand for organic carbon (Strous et al., 1999). Therefore, anammox is an environmentally friendly and energy-saving process compared to denitrification.
The 15N isotope pairing technique (IPT) has been applied to a variety of sediments to quantify nitrogen loss rates in these settings (Nielsen, 1992; Robertson et al., 2019). Slurry incubation and intact core incubations in combination with IPT are two widely used methods for studying benthic nitrogen transformation pathways (Song et al., 2016b). Slurry incubations have been used to estimate the potential rates and have advantages in discovering nitrogen loss processes in the environment (Thamdrup and Dalsgaard, 2002) as well as studying the environmental controls of these pathways; however, the natural gradients of substrates and redox in sediments were disrupted during incubations (Trimmer et al., 2006). The intact core incubations can quantify nitrogen removal processes in intact sediments and reflect the genuine benthic nitrogen transformation rates. The application of intact core incubations will enable us to fully clarify and understand the nitrogen cycle in field aquatic ecosystems.
Over the past 30 years, the introduction of isotope pairing technology has enabled numerous studies to measure anammox and denitrification using intact core incubations across a range of coastal and marine environments. These environments include intertidal wetlands (Adame et al., 2019; Liu et al., 2020), estuaries and coasts (Chen et al., 2021; Cheung et al., 2024; Deek et al., 2013; Hellemann et al., 2017), lagoons (Bernard et al., 2015; Magri et al., 2020) and oceans (Deutsch et al., 2010; Na et al., 2018). Despite decades of observations, the majority of studies on denitrification and anammox have been limited to local or regional scales. Various environmental factors, such as the availability of organic carbon (Yin et al., 2015) and nitrate (Asmala et al., 2017), dissolved oxygen (Bonaglia et al., 2013; Song et al., 2021), and temperature (Tan et al., 2022), influence these processes in coastal marine ecosystems. The modelling community has also conducted many studies on the environmental regulation of nitrogen loss (mainly denitrification) and improved the predictive parameters of denitrification (Middelburg et al., 1996; Bohlen et al., 2012; Li et al., 2024). However, according to the currently available observational data, the global patterns and drivers of sediment nitrogen loss rates remain poorly understood in coastal and marine systems.
In view of the critical role of nitrogen removal processes and the current lack of a comprehensive database on actual nitrogen loss in coastal and marine systems, we have integrated actual nitrogen loss rates, including denitrification and anammox, from published studies and constructed a dataset on nitrogen removal rates in these systems. This study provides a global-scale overview of the biogeography and potential controlling factors of denitrification and anammox in coastal and marine ecosystems. It also highlights the potential applications of this database, such as using machine learning to predict the distribution of denitrification and anammox and offering a crucial dataset for the parameterization and development of biogeochemical models.
2.1 Data compilation
Nitrogen loss rates, including denitrification and anammox measured through intact core incubations in coastal and marine ecosystems, were extracted from the literature published between 1996 and 2024. Table 1 summarizes the locations, observation numbers, core incubation methods and references of nitrogen loss rate measurements. The intact core incubations in this study include both traditional core incubations (Bonaglia et al., 2017; Cheung et al., 2024) and continuous-flow experiments (Liu et al., 2020; McTigue et al., 2016). For the continuous-flow experiments, incubations were carried out in a flow-through system where bottom water was pumped over intact cores using a multi-channel peristaltic pump, and inflow and outflow samples were collected to quantify the nitrogen process rates after the addition of 15N tracer (Gardner and McCarthy, 2009). The peer-reviewed articles compiled in this study were sourced from the Web of Science database as of June 2024. The search terms were “denitrification” or “anammox” or “nitrogen loss” or “nitrogen removal”. Given that a recent study has already summarized the data on nitrogen loss rates by slurry incubations in aquatic systems (He et al., 2025), this work selected only data in which denitrification and/or anammox rates were measured using intact core incubations with 15N isotope pairing techniques, excluding measurements derived from slurry incubations. The intact core incubation experiments were primarily conducted in dark conditions and near-in situ or in situ ambient temperatures. Photosynthetic O2 production can influence O2 penetration depth and thereby nitrate availability in sediments, interfering with denitrification rates in the nitrate reduction zone (Chen et al., 2021; Bartoli et al., 2021). In cases where nitrogen loss rates were measured under both light and dark conditions, only those measured in the dark were included to avoid photosynthesis and facilitate comparison with other studies. Measurements under light conditions have been detailed in studies reported by Bartoli et al. (2021), Chen et al. (2021), Risgaard-Petersen et al. (2004), Rysgaard et al. (1996b) and Welsh et al. (2000). Some studies have investigated the changes in nitrogen loss processes under varying oxygen concentrations (Bonaglia et al., 2013; Neubacher et al., 2011; Song et al., 2021); however, only nitrogen loss rates measured under ambient oxygen concentrations were extracted for this database. Some coastal zones are inhabited by plants and animals; whole core incubation would exclude the effect of benthic fauna or bioturbation, as the nutrient and oxygen availabilities in the core might not reflect in situ sediment characteristics. In addition, whole core incubation would exclude the effect of antibiotics addition because antibiotics addition could influence in situ nitrogen removal rates (Wan et al., 2023). Thus, studies examining the effects of meiofauna or antibiotics on nitrogen removal were not included (Bonaglia et al., 2014b; Wan et al., 2023); only rates measured without meiofauna or antibiotic additions were considered. At least one environmental variable was recorded for each selected study, and means and sample sizes had to be reported for the nitrogen removal rates. Articles that only reported nitrogen loss rates without any environmental variables were excluded. Data on total nitrogen loss rates (the sum of denitrification and anammox), denitrification rates, anammox rates and related environmental variables were collected from tables, text and/or supplementary materials and, in some cases, extracted from graphs using the Origin 2020 software. The unit conversions were performed where necessary. For example, nitrogen loss (including denitrification and anammox) rates were in µmol N m−2 h−1. When rates in the texts were displayed as mmol N m−2 d−1 or µmol N m−2 d−1, they were converted to µmol N m−2 h−1. In addition, longitude and latitude were extracted from figures from published articles if not shown in the main text.
Table 1Summary of the observations of actual nitrogen loss rates. The locations, water depth range, observation numbers, core incubation methods and references are listed.
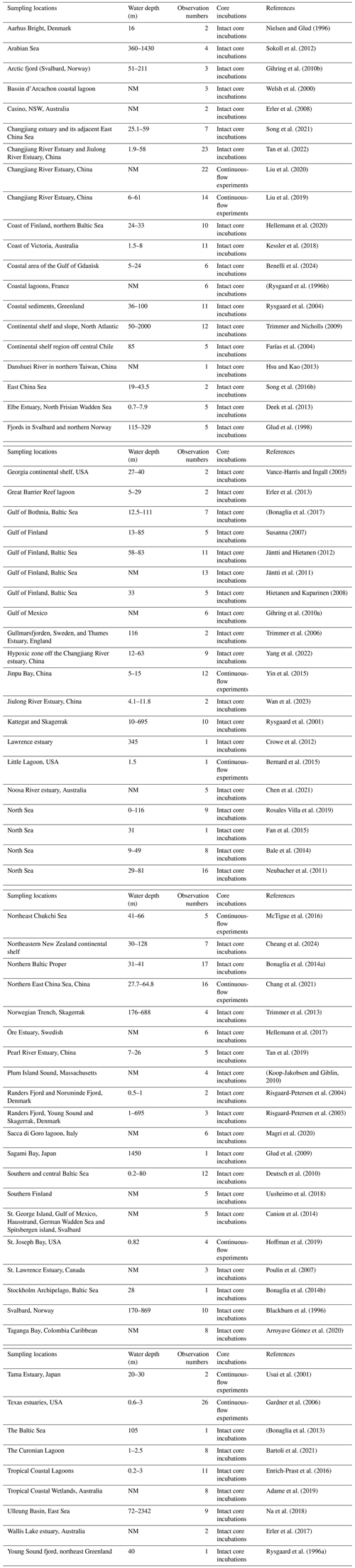
NM denotes that water depth is not mentioned.
The database includes observation details (year of sampling, month of sampling, latitude and longitude), sediment parameters and water physicochemical factors, such as sediment organic carbon, the ratios of carbon to nitrogen ( ratios) and oxygen penetration depth, as well as water salinity, depth, temperature, dissolved oxygen (DO), and ammonium and nitrate concentrations. Note that some environmental variables were not reported in the original studies. NM represents parameters that were not measured, and empty or NA indicates data not available or reported. In total, the database comprises 473, 466, 255 and 255 measurements of total nitrogen loss rates, denitrification rates, anammox rates and the relative contribution of anammox to total nitrogen loss, respectively. Authors and interested readers are welcome to contact us to indicate an error or update the data in the database.
For quality control, extreme nitrogen loss rate values were excluded from the database following Chauvenet's criterion (Glover et al., 2011), a method typically applied to normally distributed data to identify outliers whose deviation from the mean has a probability lower than . More details about Chauvenet's criterion can be found in Glover et al. (2011) and Buitenhuis et al. (2013). Very high rates of denitrification were observed in the Tama Estuary, Japan (Usui et al., 2001), a constructed wetland in Casino, NSW, Australia (Erler et al., 2008), a coastal lagoon in Sacca di Goro lagoon, Italy (Magri et al., 2020), and the Tropical Coastal Wetlands, Australia (Adame et al., 2019). For anammox, high rates were found only in a constructed wetland in Casino, NSW, Australia (Erler et al., 2008). Similarly, high values for anammox's contribution to total nitrogen loss were observed in the Changjiang River Estuary (also called Yangtze River Estuary), China (Liu et al., 2020), the Norwegian Trench, Skagerrak (Trimmer et al., 2013), and the Great Barrier Reef lagoon (Erler et al., 2013), with contributions exceeding 70 %. Observations with nitrogen loss rates of 0 or NA were excluded from the outlier analysis. For example, anammox rates of 0 were reported in the Changjiang River Estuary, China (Liu et al., 2020), the North Sea (Neubacher et al., 2011; Rosales Villa et al., 2019), the Pearl River Estuary, China (Tan et al., 2019), the Norwegian Trench, Skagerrak (Trimmer et al., 2013), and the Gulf of Finland, Baltic Sea (Jäntti et al., 2011). After excluding observations of 0 and NA (0, 8, 252 and 253 observations for total nitrogen loss rates, denitrification rates, anammox rates and anammox's contribution to total nitrogen loss), the nitrogen loss rates were natural-log transformed for further analysis.
2.2 Methods for measuring denitrification and anammox rates
Before the discovery of anammox, denitrification was regarded as the sole significant pathway responsible for nitrogen loss (Dalsgaard and Thamdrup, 2002). The 15N isotope pairing technique (IPT) was developed to quantify denitrification rates (Nielsen, 1992). In this method, the overlying water of intact sediment cores is enriched with 15NO, which is mixed with the naturally occurring 14NO. After a few hours of incubation, the denitrification products, 15N-labelled dinitrogen gas (29N2 and 30N2), are measured. Incubations to measure nitrogen loss rates have been conducted mainly in dark conditions and near-in situ or in situ ambient temperatures. After incubating for 1 h to over 96 h, the incubation is halted by injecting saturated HgCl2 or ZnCl2 saturation solution or 37 % formaldehyde. The samples are then preserved for 15N2 gas analyses through isotope ratio mass spectrometer (IRMS) or membrane inlet mass spectrometry (MIMS). Key experimental details, such as incubation conditions, temperature control, incubation time, termination and calculation references, are compiled in the database if provided in the original studies. For more detailed experimental information, refer to the corresponding references.
The production rate of unlabelled 14NO (IPTp14, also referred to as the genuine production of N2) can be calculated based on the assumption of random isotope pairing during the denitrification of the uniformly mixed NO species. The following equation is commonly used to estimate the genuine N2 production (Nielsen, 1992; Steingruber et al., 2001):
where p29N2 and p30N2 represent the total production rates of 29N2 and p30N2, respectively.
Thamdrup and Dalsgaard (2002) were the first to quantify anammox through anaerobic slurry incubations in natural environments, discovering that anammox could account for more than 60 % of the total N2 production. This highlighted the significant role of anammox in nitrogen removal. Following this, Risgaard-Petersen et al. (2003) proposed a modification to the traditional IPT, allowing for more accurate quantification of true N2 production in environments where anammox and denitrification coexist. This revision also enables the distinction between N2 produced by anammox and denitrification. The revised IPT (rIPT) follows the same procedure as the classical IPT, with 15NO added to the overlying water of intact sediment cores, though the calculation process is more complex. The following equations are commonly used to estimate the actual N2 production (rIPTp14) and denitrification (p14DEN) as well as anammox (p14ANA) (Risgaard-Petersen et al., 2003; Trimmer and Nicholls, 2009; Trimmer et al., 2006). The total N2 production rate is the sum of the denitrification and anammox rates.
In these equations, p29N2 and p30N2 are the total production rates of 29N2 and p30N2, respectively, and r14 represents the ratio of 14NO to 15NO in the nitrate reduction zone. There are three different methods to estimate r14, with detailed explanations available in Trimmer et al. (2006).
Subsequently, Hsu and Kao (2013) revised the rIPT method to incorporate both N2O production and anammox, enabling the determination of the absolute rate of each nitrogen loss pathway, including denitrification, anammox and N2O production from denitrification. Denitrification and anammox measurements based on the method of Hsu and Kao (2013) are included in this database, whereas data on the true N2O production rate have not been included.
Regarding the aforementioned calculation methods, Salk et al. (2017) have systematically reviewed different methods for quantifying nitrogen loss rates and illustrated their differences with diagrams distinguishing different processes, providing valuable guidance for researchers interested in this field. Therefore, interested researchers can refer to their article.
3.1 Overview of the database
Overall, there are 473, 466 and 255 measurements for total nitrogen loss, denitrification and anammox, respectively (Fig. 1). Denitrification and anammox have been measured simultaneously for 255 observations. The observations of nitrogen loss rates are primarily distributed along the East Coast of the United States, the Baltic Sea, the Eastern Coast of China, the Eastern Coast of Australia, and polar regions of the Northern Hemisphere (Fig. 1a). Before 2000, nitrogen loss measurements were predominantly focused on denitrification, while both denitrification and anammox rates have been measured concurrently since 2000 (Fig. 1b). Notably, more observations were recorded in 2011 and 2017. The studies in 2011 were mainly conducted in the Changjiang estuary and its adjacent East China Sea (Song et al., 2021), the Jinpu Bay, China (Yin et al., 2015), the North Sea (Bale et al., 2014), the Northern Baltic Proper (Bonaglia et al., 2014a), and the hypoxic zone off the Changjiang River estuary, China (Yang et al., 2022). In 2017, high observations were found in the Northern East China Sea, China (Chang et al., 2021), the Changjiang River Estuary, China (Liu et al., 2020; Liu et al., 2019; Tan et al., 2022), the Coast of Victoria, Australia (Kessler et al., 2018), and the Jiulong River Estuary, China (Tan et al., 2022).
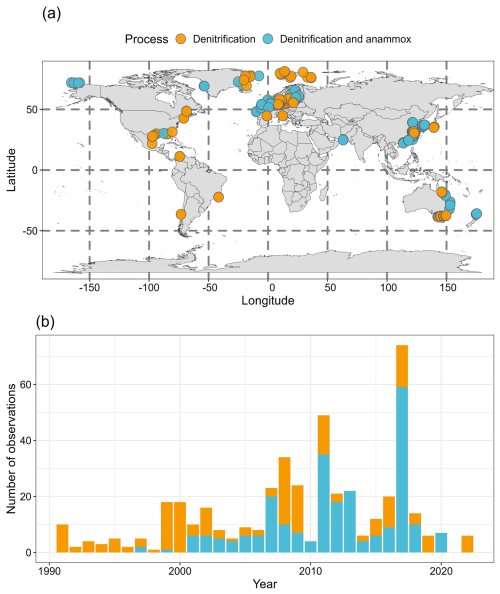
Figure 1Map showing the sampling site distribution of nitrogen loss rate measurements (a) and the number of rate observations each year (b). Orange solid points denote that only denitrification rates were measured. Cyan solid points denote that both denitrification and anammox rates were measured. Publisher's remark: please note that the above figure contains disputed territories.
3.2 Distribution of denitrification
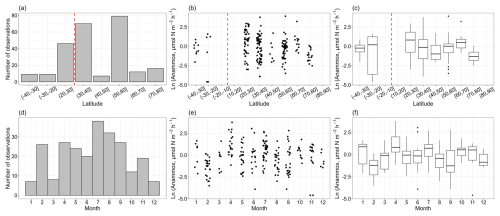
In total, the vast majority of nitrogen loss rate measurements were conducted in the Northern Hemisphere, and data in the Southern Hemisphere were limited (Fig. 2a, b, c). The low and middle latitudes of the Northern Hemisphere have a large body of observations, especially in the 20–30, 30–40 and 50–60° N latitude bands. Denitrification rates ranged from 0.04 to 750 µmol N m−2 h−1, with a median value of 7.72 ± 4.30 µmol N m−2 h−1. There is a decreasing trend in the denitrification rates with latitude in the Northern Hemisphere, though the observations at high latitude are still limited. The measurements of denitrification were primarily conducted between April and September (Fig. 2d, e, f). On a global scale, no clear seasonal pattern for denitrification rates was observed.
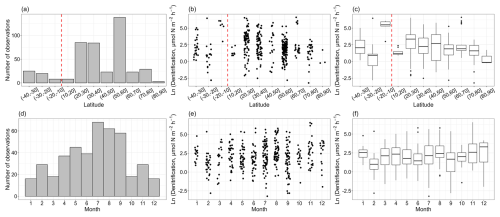
Figure 2The observation numbers for denitrification (a, d) and denitrification rates (b, c, e, f) with the corresponding latitudinal bands and months. A vertical dashed red line delimits the Southern Hemisphere and the Northern Hemisphere. The box plots show the median, interquartile range and outliers for each latitudinal band and month.
3.3 Distribution of anammox
From a latitude perspective, the distribution of anammox rates closely mirrored that of denitrification, with the majority of observations concentrated in the 20–30, 30–40 and 50–60° N latitude bands (Fig. 3a, b, c). However, compared to denitrification, there were fewer anammox observations. Anammox rates spanned from 0.01 to 48.94 µmol N m−2 h−1, with a median value of 1.00 ± 0.39 µmol N m−2 h−1. Similar to denitrification, anammox rates also showed a decreasing trend with increasing latitude in the Northern Hemisphere. Numerous anammox measurements were conducted between April and September, consistent with the timing of denitrification measurements (Fig. 3d, e, f). Additionally, February saw a high number of anammox observations, and these observations were predominantly conducted at the north East China Sea (Chang et al., 2021), the Changjiang estuary (Liu et al., 2019) and the Northeastern New Zealand continental shelf regions (Cheung et al., 2024). On a global scale, there was no clear seasonal pattern for anammox rates.
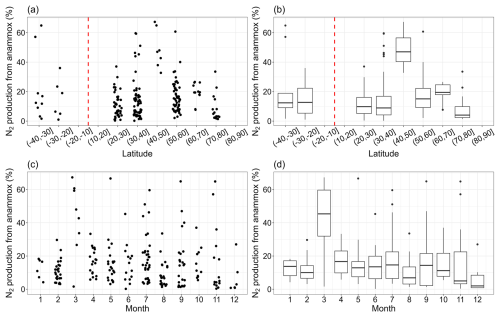
3.4 Distribution of contributions of anammox to total N2 production
The relative importance of anammox to total N2 production increased first and then decreased, peaking in the 40–50° N latitudinal band in the Northern Hemisphere, although data points in this band were limited (Fig. 4). The contribution of anammox to total N2 production varied from 0.22 % to 67.33 %, with a median value of 12.29 %. The highest value (67.33 %) was recorded at a site on the North Atlantic continental slope at a depth of 2000 m (Trimmer and Nicholls, 2009), where anammox accounted for the majority of nitrogen removal. There were no significant monthly changes in the relative importance of anammox to total nitrogen loss, except for March, when anammox contributed a notably high percentage. High values in March were observed in the Ulleung Basin, the East Sea, and the continental shelf and slope, North Atlantic (Na et al., 2018; Trimmer and Nicholls, 2009), where the stations were characterized by low nitrate levels or deep water. These environmental conditions may inhibit denitrification, thereby increasing the relative contribution of anammox to nitrogen loss. It is worth noting that the rate observations in March were mainly distributed in certain regions. Thus, the extrapolations of the relative importance of anammox in coastal marine ecosystems at the monthly level using this result should be cautious. More observation data for other regions are needed in the future.
3.5 Control factors on denitrification and anammox rates
The variations in denitrification rates and anammox rates were compared against several environmental variables, including sediment organic carbon, the ratios of carbon to nitrogen ( ratios) and oxygen penetration depth, as well as water depth, temperature, salinity, dissolved oxygen, and ammonium and nitrate concentrations. This comparison was conducted to evaluate the main controlling factors of nitrogen loss rates.
There was no significant relationship between denitrification rates and the contents of sediment organic carbon (p> 0.05; Fig. 5a). Heterotrophic denitrification is primarily carried out by facultative anaerobic heterotrophs (Devol, 2015), which use organic carbon as an electron donor and energy source. Therefore, higher organic carbon levels might be expected to promote denitrification (Damashek and Francis, 2018). However, no such relationship was observed in this dataset. Denitrification rates increased with the sediment carbon-to-nitrogen ratio (r= 0.32, p<0.01; Fig. 5b). The ratios can indicate the reactivity of sediment organic material, with lower values generally representing more reactive organic matter (Cheung et al., 2024; Erler et al., 2013). Typically, high denitrification rates are associated with sediments that have lower ratios. However, in this analysis, the opposite trend was observed. One possible explanation is that microbial communities may adapt to use organic matter typically encountered, though the organic matter is not labile (Salk et al., 2017). Denitrification rates showed a weak negative correlation with oxygen penetration depth (r= −0.29, p< 0.05; Fig. 5c), as greater O2 penetration may be adverse to the occurrence of denitrification (Cheung et al., 2024). Denitrification rates also decreased with water depth (r= −0.26, p< 0.01; Fig. 5d), with most observations occurring at depths shallower than 250 m. Denitrification was positively correlated with higher water temperatures (r= 0.38, p < 0.01; Fig. 5e) and negatively correlated with salinity (r= −0.15, p < 0.01; Fig. 5f), with most rates falling within two salinity ranges (0–10 and 30–40). Samples that had a salinity greater than 40 were collected in hypersaline lagoons of tropical regions (Enrich-Prast et al., 2016). The relationship between denitrification and salinity across coastal environments has been summarized by Torregrosa-Crespo et al. (2023) and will not be further elaborated here. There was a weak negative relationship between denitrification rates and dissolved oxygen concentrations (r=-0.23, p < 0.01; Fig. 5g). Overall, higher denitrification rates were recorded in areas with high nitrate concentrations (r= 0.16, p < 0.01; Fig. 5h), suggesting the importance of the nitrate substrate in regulating denitrification, though some high rates were also observed at sites with low nitrate levels. No significant correlation was found between denitrification rates and ammonium concentrations (p > 0.05; Fig. 5i).
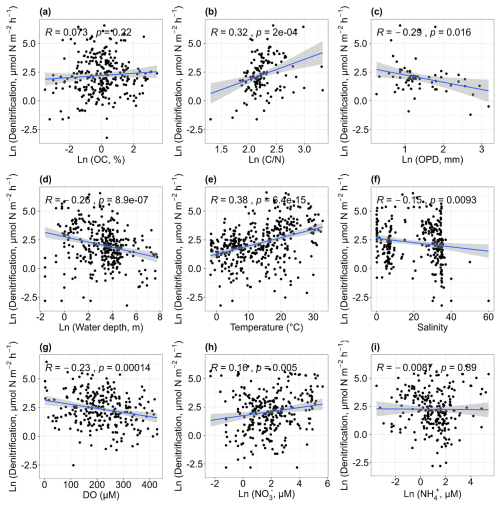
Figure 5Relationships between denitrification rates and organic carbon [OC, a], carbon–nitrogen ratios [, b], oxygen penetration depth [OPD, c], water depth (d), temperature (e), salinity (f), dissolved oxygen [DO, g], nitrate concentrations [NO, h] and ammonium concentrations [NH, i].
Anammox rates showed a weak positive correlation with sediment organic carbon (r= 0.16, p < 0.05; Fig. 6a). Although anammox is an autotrophic process that does not require organic carbon as an electron donor (Salk et al., 2017), some studies have reported links between sediment organic carbon content and anammox rates. For example, studies in subtropical mangrove sediments (Meyer et al., 2005) and the Thames estuary (Trimmer et al., 2003) found that higher organic matter stimulated anammox. This correlation may be due to enhanced mineralization leading to increased ammonium production, which indirectly stimulates anammox (Damashek and Francis, 2018), as sediment organic carbon can serve as a proxy for organic carbon mineralization (Song et al., 2016a). Similar to denitrification, high anammox rates were observed at sites with elevated ratios (r= 0.33, p < 0.01; Fig. 6b). We infer that, to some extent, the coupling of denitrification and anammox may account for this relation. As mentioned above, denitrification stimulated with higher ratios; the decomposition of organic matter could provide a substrate for anammox, thereby promoting anammox. More studies are needed to reveal the influencing mechanisms of ratios on anammox. No clear trend was found between anammox rates and oxygen penetration depth (p > 0.05; Fig. 6c), and high anammox rates were observed in shallow waters (p > 0.05; Fig. 6d). Anammox rates showed a weak positive correlation with temperature (r= 0.19, p < 0.01; Fig. 6e). While several studies have suggested that low temperatures could favour anammox (Dalsgaard and Thamdrup, 2002; Rysgaard et al., 2004; Tan et al., 2020), these studies primarily measured anammox potential using anaerobic slurry incubations. Contrary to previous findings, our study showed that actual anammox rates increased with rising temperatures, suggesting a discrepancy between the effects of temperature on actual and potential anammox rates. Future research is needed to investigate the underlying mechanisms for these inconsistent results. Anammox rates decreased with increasing salinity (r= −0.38, p < 0.01; Fig. 6f) and showed no significant relationship with dissolved oxygen (p > 0.05; Fig. 6g). A weak positive correlation was observed between anammox rates and nitrate concentration (r= 0.41, p < 0.01; Fig. 6h), highlighting the importance of substrates in regulating anammox. Although anammox uses nitrite as an electron acceptor rather than nitrate (van de Graaf et al., 1995), nitrate reduction can produce nitrite, which promotes anammox activity. No relationship was found between anammox rates and ammonium concentration (p > 0.05; Fig. 6i).
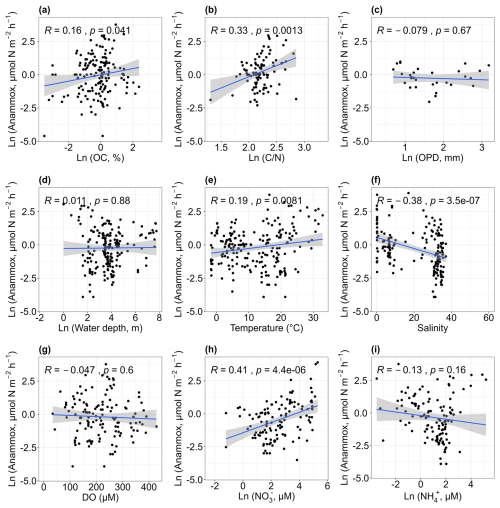
Figure 6Relationships between anammox rates and organic carbon [OC, a], carbon–nitrogen ratios [, b], oxygen penetration depth [OPD, c], water depth (d), temperature (e), salinity (f), dissolved oxygen [DO, g], nitrate concentrations [NO, h] and ammonium concentrations [NH, i].
Through the correlation analysis of global-scale compiled data, we identified that sediment ratios, oxygen penetration depth, water depth, temperature, salinity, dissolved oxygen and nitrate concentrations were the main factors regulating denitrification rates, whereas sediment organic carbon, ratios, temperature, salinity and nitrate concentrations primarily controlled anammox rates (Figs. 5 and 6).
Other factors, such as iron, manganese and sulfide, although not included in the database, can also influence denitrification and anammox rates. For example, Fe oxides were observed to be positively correlated with denitrification rates in the Jinpu Bay, China (Yin et al., 2015). The mechanism may be that ferrous iron can supply an electron donor for nitrate, thereby promoting denitrification. Anschutz et al. (2000) found that manganese dioxides could also serve as electron donors for denitrification. Deng et al. (2015) showed a positive relationship between denitrification rates and sulfide concentrations in the Changjiang estuary sediments, revealing that sulfide can act as an energy source for denitrification. In contrast, evidence has shown that sulfide exerts inhibitory effects on nitrogen removal in coastal sediments by inhibiting the metabolism of denitrifying microorganisms (Aelion and Warttinger, 2010). Thus, the impact of sulfide on denitrification remains controversial. For anammox, a study found that sulfide could affect anammox activity. Yin et al. (2015) found that anammox rates were positively correlated with sulfide concentrations. This phenomenon is likely attributed to sulfide-induced nitrite accumulation during incomplete denitrification processes, where sulfide inhibits the activity of nitric oxide reductase and nitrous oxide reductase, thereby enhancing anammox activity. Under anaerobic conditions, ammonium oxidation can be coupled with the reduction of ferric iron, sulfate and Mn(IV)-oxides. For example, Rios-Del Toro et al. (2018) confirmed that ammonium oxidation was associated with ferric iron and sulfate reduction under anaerobic conditions, thereby stimulating nitrogen loss in marine sediments. Evidence shows that ammonium loss is coupled with Fe(III) and Mn(IV) reduction in coastal environments (Samperio-Ramos et al., 2024), demonstrating the crucial roles of metal oxides in removing reactive nitrogen.
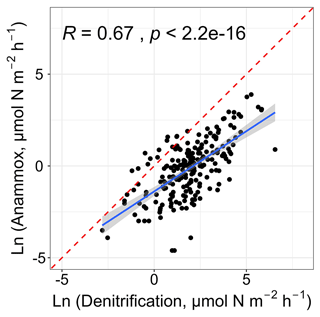
Liu et al. (2020) have examined the spatiotemporal changes of in situ nitrogen loss processes in intertidal wetlands of the Yangtze Estuary and found that denitrification was linked to anammox, implying the coupling of denitrification and anammox on a local scale. Consistent with their findings, this work also found that denitrification was positively correlated with anammox (r= 0.67, p < 0.01; Fig. 7). A majority of denitrifying bacteria are heterotrophic, and the decomposition of organic matter is accompanied by the production of ammonium (Devol, 2015), supplying substrates for anammox. Thus, the positive relationship may suggest the tight coupling of these two nitrogen removal pathways on a global scale.
3.6 Drivers behind the contribution of anammox to total nitrogen loss
We performed simple correlation analysis between the contribution of anammox to total N2 production (ra) and environmental parameters (Fig. 8). There was a positive correlation between ra and water depth (r= 0.59, p < 0.01; Fig. 8d). Similar findings were found on the Northeastern New Zealand continental shelf (Cheung et al., 2024) and the continental shelf and slope, North Atlantic (Trimmer and Nicholls, 2009). The increased importance of anammox can be attributed to the significant attenuation of denitrification with depth, as the availability of organic carbon essential for heterotrophic denitrification generally decreases with water depth (Thamdrup, 2012). In addition to water depth, other factors such as oxygen penetration depth, ratios and temperature may also influence the relative importance of anammox. The ra was positively correlated with oxygen penetration depth (r= 0.7, p < 0.01; Fig. 8c). As previously mentioned, denitrification decreases as oxygen penetration depth increases, likely increasing the relative importance of anammox indirectly. Conversely, ra showed a decreasing trend with elevated ratios (r= −0.35, p < 0.01; Fig. 8b). High ratios may promote denitrification more significantly than anammox because both processes tend to improve with increasing ratios, leading to a decrease in the relative importance of anammox at sites with high ratios. Additionally, ra was negatively correlated with temperature (r= −0.29, p < 0.01; Fig. 8e), indicating that denitrification is stimulated at higher temperatures compared to anammox. Temperature-controlled experiments have confirmed that denitrification has a greater optimal temperature than anammox (Canion et al., 2014; Tan et al., 2020). No correlations were found between ra and other environmental factors, including sediment organic carbon, water salinity, dissolved oxygen and nitrate and ammonium concentrations (all p > 0.05; Fig. 8a, f, g, h, i). Based on the simple correlation analysis of global-scale compiled data, we identified that sediment ratios, oxygen penetration depth, water depth and temperature were the primary factors governing the relative contribution of anammox to total nitrogen loss (Fig. 8).
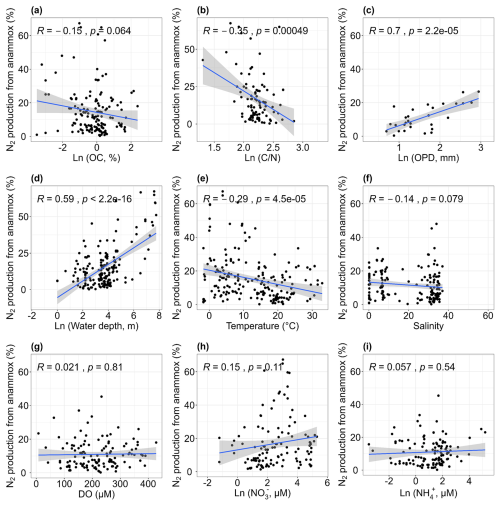
Figure 8Relationships between the relative contribution of anammox to total N2 production and organic carbon [OC, a], carbon–nitrogen ratios [, b], oxygen penetration depth [OPD, c], water depth (d), temperature (e), salinity (f), dissolved oxygen [DO, g], nitrate concentrations [NO, h] and ammonium concentrations [NH, i].
This database serves as a valuable resource for the broad scientific communities that are interested in nitrogen cycle processes within coastal and marine ecosystems, particularly those focusing on denitrification and anammox. The data are made accessible as a basic database that will lead to a deeper understanding and generate new scientific insights into the nitrogen cycles at the global scale. Potential applications of this database include the following: (1) It can serve as a reference for comparing denitrification and anammox rates across different spatial scales, including local, regional and global scales, or across different habitats, such as coastal wetland, estuary, lagoon and ocean, in future studies. (2) It can be used to identify and compare the controlling factors of denitrification and anammox at various spatial scales. Note that environmental variables have missing values, which limits our analysis of environmental factors affecting nitrogen loss rates. To better study the environmental controls, these missing values can be filled in using the multivariate imputation with the random forests method (Hou et al., 2021). (3) The database can be used to predict the global biogeography of denitrification and anammox in coastal and marine systems through machine learning methods. For example, by integrating potential key factors of nitrogen removal processes into machine learning architectures, future studies can develop spatially predictive models for global nitrogen loss rates by following Laffitte et al. (2025) and Ling et al. (2025). (4) It can provide essential data for the parameterization, validation and enhancement of Earth system biogeochemical models. The previous model considered constraint parameters such as nitrate, dissolved oxygen, chlorophyll and phosphate content (Middelburg et al., 1996; Bohlen et al., 2012; Li et al., 2024); the other parameters provided in this dataset can supply new parameter supplements for the development of biogeochemical model. (5) It can guide future observations. More studies are needed in areas and months with limited observation data on nitrogen loss rates to deepen our understanding of the nitrogen cycle worldwide. Additionally, when studying nitrogen loss rates, particular attention should be paid to enhancing the monitoring of multiple environmental parameters.
The data used in this study are openly available from the Figshare repository at https://doi.org/10.6084/m9.figshare.27745770.v3 (Chang et al., 2024).
We compiled and presented a global database of denitrification and anammox measurements obtained from core incubation experiments in coastal and marine sediments. To our knowledge, no efforts have been made to compile actual nitrogen loss rates and associated environmental factors in coastal and marine regions on a global scale. This database offers valuable insights into the spatiotemporal variations and potential controlling factors of denitrification and anammox, along with the contribution of anammox to total N2 production. The establishment of this global database on denitrification and anammox in coastal and marine sediments provides a critical foundation for advancing nitrogen cycle research and generating novel insights. This database enables the comparison of these two nitrogen loss processes, evaluation of the environmental controls across spatial scales (local to global), prediction of the global biogeography of denitrification and anammox, and the parameterization and development of biogeochemical models, and it can be used to guide the direction of observations in the future.
SJK and EHT conceived the research. YKC and EHT compiled the data. YKC, EHT, DZG, CL and SJK participated in the data analysis. All co-authors contributed to the writing and reviewing of this paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank the authors for their contributions to the data used in this database. Thanks also go to the editors and reviewers for their constructive comments and suggestions, which helped us to improve this paper greatly.
This work was supported by the National Natural Science Foundation of China (grant no. 42276043), the Hainan Provincial Natural Science Foundation of China (grant no. 623RC456), the Collaborative Innovation Center of Marine Science and Technology in Hainan University (grant no. XTCX2022HYC19), the Innovational Fund for Scientific and Technological Personnel of Hainan Province (grant no. KJRC2023B04), and the Shandong Provincial Natural Science Foundation of China (grant no. ZR2023QD103).
This paper was edited by Xingchen (Tony) Wang and reviewed by two anonymous referees.
Adame, M. F., Roberts, M. E., Hamilton, D. P., Ndehedehe, C. E., Reis, V., Lu, J., Griffiths, M., Curwen, G., and Ronan, M.: Tropical Coastal Wetlands Ameliorate Nitrogen Export During Floods, Front. Mar. Sci., 6, 671, https://doi.org/10.3389/fmars.2019.00671, 2019.
Aelion, C. M. and Warttinger, U.: Sulfide Inhibition of Nitrate Removal in Coastal Sediments, Estuaries Coasts, 33, 798–803, https://doi.org/10.1007/s12237-010-9275-4, 2010.
Anschutz, P., Sundby, B., Lefrançois, L., Luther, G. W., and Mucci, A.: Interactions between metal oxides and species of nitrogen and iodine in bioturbated marine sediments, Geochim. Cosmochim. Ac., 64, 2751–2763, https://doi.org/10.1016/S0016-7037(00)00400-2, 2000.
Arroyave Gómez, D. M., Gallego Suárez, D., Bartoli, M., and Toro-Botero, M.: Spatial and seasonal variability of sedimentary features and nitrogen benthic metabolism in a tropical coastal area (Taganga Bay, Colombia Caribbean) impacted by a sewage outfall, Biogeochemistry, 150, 85–107, https://doi.org/10.1007/s10533-020-00689-0, 2020.
Asmala, E., Carstensen, J., Conley, D. J., Slomp, C. P., Stadmark, J., and Voss, M.: Efficiency of the coastal filter: Nitrogen and phosphorus removal in the Baltic Sea, Limnol. Oceanogr., 62, S222–S238, https://doi.org/10.1002/lno.10644, 2017.
Bale, N. J., Villanueva, L., Fan, H., Stal, L. J., Hopmans, E. C., Schouten, S., and Sinninghe Damsté, J. S.: Occurrence and activity of anammox bacteria in surface sediments of the southern North Sea, FEMS Microbiol. Ecol., 89, 99–110, https://doi.org/10.1111/1574-6941.12338, 2014.
Bartoli, M., Nizzoli, D., Zilius, M., Bresciani, M., Pusceddu, A., Bianchelli, S., Sundbäck, K., Razinkovas-Baziukas, A., and Viaroli, P.: Denitrification, Nitrogen Uptake, and Organic Matter Quality Undergo Different Seasonality in Sandy and Muddy Sediments of a Turbid Estuary, Front. Microbiol., 11, 612700, https://doi.org/10.3389/fmicb.2020.612700, 2021.
Benelli, S., Bartoli, M., Magri, M., Brzana, R., Kendzierska, H., Styrcz-Olesiak, K., and Janas, U.: Spatial and seasonal pattern of microbial nitrate reduction in coastal sediments in the Vistula River plume area, Gulf of Gdańsk, Front. Mar. Sci., 11, 1333707, https://doi.org/10.3389/fmars.2024.1333707, 2024.
Bernard, R. J., Mortazavi, B., and Kleinhuizen, A. A.: Dissimilatory nitrate reduction to ammonium (DNRA) seasonally dominates NO reduction pathways in an anthropogenically impacted sub-tropical coastal lagoon, Biogeochemistry, 125, 47–64, https://doi.org/10.1007/s10533-015-0111-6, 2015.
Blackburn, T. H., Hall, P. O. J., Hulth, S., and Landén, A.: Organic-N loss by efflux and burial associated with a low efflux of inorganic N and with nitrate assimilation in Arctic sediments (Svalbard, Norway), Mar. Ecol. Prog. Ser., 141, 283–293, https://doi.org/10.3354/meps141283, 1996.
Bohlen, L., Dale, A. W., and Wallmann, K.: Simple transfer functions for calculating benthic fixed nitrogen losses and regeneration ratios in global biogeochemical models, Global Biogeochem. Cy., 26, GB3029, https://doi.org/10.1029/2011GB004198, 2012.
Bonaglia, S., Bartoli, M., Gunnarsson, J. S., Rahm, L., Raymond, C., Svensson, O., Shakeri Yekta, S., and Brüchert, V.: Effect of reoxygenation and Marenzelleria spp. bioturbation on Baltic Sea sediment metabolism, Mar. Ecol. Prog. Ser., 482, 43–55, https://doi.org/10.3354/meps10232, 2013.
Bonaglia, S., Deutsch, B., Bartoli, M., Marchant, H. K., and Brüchert, V.: Seasonal oxygen, nitrogen and phosphorus benthic cycling along an impacted Baltic Sea estuary: regulation and spatial patterns, Biogeochemistry, 119, 139–160, https://doi.org/10.1007/s10533-014-9953-6, 2014a.
Bonaglia, S., Nascimento, F. J. A., Bartoli, M., Klawonn, I., and Brüchert, V.: Meiofauna increases bacterial denitrification in marine sediments, Nat. Commun., 5, 5133, https://doi.org/10.1038/ncomms6133, 2014b.
Bonaglia, S., Hylén, A., Rattray, J. E., Kononets, M. Y., Ekeroth, N., Roos, P., Thamdrup, B., Brüchert, V., and Hall, P. O. J.: The fate of fixed nitrogen in marine sediments with low organic loading: an in situ study, Biogeosciences, 14, 285–300, https://doi.org/10.5194/bg-14-285-2017, 2017.
Buitenhuis, E. T., Vogt, M., Moriarty, R., Bednaršek, N., Doney, S. C., Leblanc, K., Le Quéré, C., Luo, Y.-W., O'Brien, C., O'Brien, T., Peloquin, J., Schiebel, R., and Swan, C.: MAREDAT: towards a world atlas of MARine Ecosystem DATa, Earth Syst. Sci. Data, 5, 227–239, https://doi.org/10.5194/essd-5-227-2013, 2013.
Canfield, D. E., Glazer, A. N., and Falkowski, P. G.: The Evolution and Future of Earth's Nitrogen Cycle, Science, 330, 192–196, https://doi.org/10.1126/science.1186120, 2010.
Canion, A., Kostka, J. E., Gihring, T. M., Huettel, M., van Beusekom, J. E. E., Gao, H., Lavik, G., and Kuypers, M. M. M.: Temperature response of denitrification and anammox reveals the adaptation of microbial communities to in situ temperatures in permeable marine sediments that span 50° in latitude, Biogeosciences, 11, 309–320, https://doi.org/10.5194/bg-11-309-2014, 2014.
Chang, Y., Yin, G., Hou, L., Liu, M., Zheng, Y., Han, P., Dong, H., Liang, X., Gao, D., and Liu, C.: Nitrogen removal processes coupled with nitrification in coastal sediments off the north East China Sea, J. Soils Sediments, 21, 3289–3299, https://doi.org/10.1007/s11368-021-02964-5, 2021.
Chang, Y., Tan, E., Gao, D., Liu, C., Zhang, Z., Huang, Z., Liu, J., Han, Y., Xu, Z., Chen, B., and Kao, S.-J.: Global database of actual nitrogen loss rates in coastal and marine sediments, Figshare [data set], https://doi.org/10.6084/m9.figshare.27745770.v3, 2024.
Chen, J.-J., Erler, D. V., Wells, N. S., Huang, J., Welsh, D. T., and Eyre, B. D.: Denitrification, anammox, and dissimilatory nitrate reduction to ammonium across a mosaic of estuarine benthic habitats, Limnol. Oceanogr., 66, 1281–1297, https://doi.org/10.1002/lno.11681, 2021.
Cheung, H. L. S., Hillman, J. R., Pilditch, C. A., Savage, C., Santos, I. R., Glud, R. N., Nascimento, F. J. A., Thrush, S. F., and Bonaglia, S.: Denitrification, anammox, and DNRA in oligotrophic continental shelf sediments, Limnol. Oceanogr., 69, 621–637, https://doi.org/10.1002/lno.12512, 2024.
Crowe, S. A., Canfield, D. E., Mucci, A., Sundby, B., and Maranger, R.: Anammox, denitrification and fixed-nitrogen removal in sediments from the Lower St. Lawrence Estuary, Biogeosciences, 9, 4309–4321, https://doi.org/10.5194/bg-9-4309-2012, 2012.
Cui, S., Shi, Y., Groffman, P. M., Schlesinger, W. H., and Zhu, Y.-G.: Centennial-scale analysis of the creation and fate of reactive nitrogen in China (1910–2010), P. Natl. Acad. Sci. USA, 110, 2052–2057, https://doi.org/10.1073/pnas.1221638110, 2013.
Dai, M., Zhao, Y., Chai, F., Chen, M., Chen, N., Chen, Y., Cheng, D., Gan, J., Guan, D., Hong, Y., Huang, J., Lee, Y., Leung, K. M. Y., Lim, P. E., Lin, S., Lin, X., Liu, X., Liu, Z., Luo, Y.-W., Meng, F., Sangmanee, C., Shen, Y., Uthaipan, K., Wan Talaat, W. I. A., Wan, X. S., Wang, C., Wang, D., Wang, G., Wang, S., Wang, Y., Wang, Y., Wang, Z., Wang, Z., Xu, Y., Yang, J.-Y. T., Yang, Y., Yasuhara, M., Yu, D., Yu, J., Yu, L., Zhang, Z., and Zhang, Z.: Persistent eutrophication and hypoxia in the coastal ocean, Cambridge Prisms: Coastal Futures, 1, 1–28, https://doi.org/10.1017/cft.2023.7, 2023.
Dalsgaard, T. and Thamdrup, B.: Factors Controlling Anaerobic Ammonium Oxidation with Nitrite in Marine Sediments, Appl. Environ. Microb., 68, 3802–3808, https://doi.org/10.1128/AEM.68.8.3802-3808.2002, 2002.
Damashek, J. and Francis, C. A.: Microbial Nitrogen Cycling in Estuaries: From Genes to Ecosystem Processes, Estuaries Coasts, 41, 626–660, https://doi.org/10.1007/s12237-017-0306-2, 2018.
Deek, A., Dähnke, K., van Beusekom, J., Meyer, S., Voss, M., and Emeis, K.: N2 fluxes in sediments of the Elbe Estuary and adjacent coastal zones, Mar. Ecol. Prog. Ser., 493, 9-21, https://doi.org/10.3354/meps10514, 2013.
Deng, D., He, G., Ding, B., Liu, W., Yang, Z., and Ma, L.: Denitrification dominates dissimilatory nitrate reduction across global natural ecosystems, Glob. Change Biol., 30, e17256, https://doi.org/10.1111/gcb.17256, 2024.
Deng, F., Hou, L., Liu, M., Zheng, Y., Yin, G., Li, X., Lin, X., Chen, F., Gao, J., and Jiang, X.: Dissimilatory nitrate reduction processes and associated contribution to nitrogen removal in sediments of the Yangtze Estuary, J. Geophys. Res.-Biogeo., 120, 1521–1531, https://doi.org/10.1002/2015JG003007, 2015.
Deutsch, B., Forster, S., Wilhelm, M., Dippner, J. W., and Voss, M.: Denitrification in sediments as a major nitrogen sink in the Baltic Sea: an extrapolation using sediment characteristics, Biogeosciences, 7, 3259–3271, https://doi.org/10.5194/bg-7-3259-2010, 2010.
Devol, A. H.: Denitrification, Anammox, and N2 Production in Marine Sediments, Annu. Rev. Mar. Sci., 7, 403–423, https://doi.org/10.1146/annurev-marine-010213-135040, 2015.
Enrich-Prast, A., Figueiredo, V., Esteves, F. D. A., and Nielsen, L. P.: Controls of Sediment Nitrogen Dynamics in Tropical Coastal Lagoons, PloS One, 11, e0155586, https://doi.org/10.1371/journal.pone.0155586, 2016.
Erler, D. V., Eyre, B. D., and Davison, L.: The Contribution of Anammox and Denitrification to Sediment N2 Production in a Surface Flow Constructed Wetland, Environ. Sci. Technol., 42, 9144–9150, https://doi.org/10.1021/es801175t, 2008.
Erler, D. V., Trott, L. A., Alongi, D. M., and Eyre, B. D.: Denitrification, anammox and nitrate reduction in sediments of the southern Great Barrier Reef lagoon, Mar. Ecol. Prog. Ser., 478, 57–70, https://doi.org/10.3354/meps10040, 2013.
Erler, D. V., Welsh, D. T., Bennet, W. W., Meziane, T., Hubas, C., Nizzoli, D., and Ferguson, A. J. P.: The impact of suspended oyster farming on nitrogen cycling and nitrous oxide production in a sub-tropical Australian estuary, Estuarine, Coastal Shelf Sci., 192, 117–127, https://doi.org/10.1016/j.ecss.2017.05.007, 2017.
Fan, H., Bolhuis, H., and Stal, L. J.: Drivers of the dynamics of diazotrophs and denitrifiers in North Sea bottom waters and sediments, Front. Microbiol., 6, 738, https://doi.org/10.3389/fmicb.2015.00738, 2015.
Farías, L., Graco, M., and Ulloa, O.: Temporal variability of nitrogen cycling in continental-shelf sediments of the upwelling ecosystem off central Chile, Deep-Sea Res. Pt. II, 51, 2491–2505, https://doi.org/10.1016/j.dsr2.2004.07.029, 2004.
Gardner, W. S. and McCarthy, M. J.: Nitrogen dynamics at the sediment–water interface in shallow, sub-tropical Florida Bay: why denitrification efficiency may decrease with increased eutrophication, Biogeochemistry, 95, 185–198, https://doi.org/10.1007/s10533-009-9329-5, 2009.
Gardner, W. S., McCarthy, M. J., An, S., Sobolev, D., Sell, K. S., and Brock, D.: Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries, Limnol. Oceanogr., 51, 558–568, https://doi.org/10.4319/lo.2006.51.1_part_2.0558, 2006.
Gihring, T. M., Lavik, G., Kuypers, M. M. M., and Kostka, J. E.: Direct determination of nitrogen cycling rates and pathways in Arctic fjord sediments (Svalbard, Norway), Limnol. Oceanogr., 55, 740–752, https://doi.org/10.4319/lo.2010.55.2.0740, 2010a.
Gihring, T. M., Canion, A., Riggs, A., Huettel, M., and Kostk, J. E.: Denitrification in shallow, sublittoral Gulf of Mexico permeable sediments, Limnol. Oceanogr., 55, 43–54, https://doi.org/10.4319/lo.2010.55.1.0043, 2010b.
Glover, D. M., Jenkins, W. J., and Doney, S. C.: Modeling Methods for Marine Science, Cambridge University Press, Cambridge, https://doi.org/10.1017/CBO9780511975721, 2011.
Glud, R. N., Holby, O., Hoffmann, F., and Canfield, D. E.: Benthic mineralization and exchange in Arctic sediments (Svalbard, Norway), Mar. Ecol. Prog. Ser., 173, 237–251, https://doi.org/10.3354/meps173237, 1998.
Glud, R. N., Thamdrup, B., Stahl, H., Wenzhoefer, F., Glud, A., Nomaki, H., Oguri, K., Revsbech, N. P., and Kitazato, H.: Nitrogen cycling in a deep ocean margin sediment (Sagami Bay, Japan), Limnol. Oceanogr., 54, 723–734, https://doi.org/10.4319/lo.2009.54.3.0723, 2009.
He, G., Deng, D., Delgado-Baquerizo, M., Liu, W., and Zhang, Q.: Global Relative Importance of Denitrification and Anammox in Microbial Nitrogen Loss Across Terrestrial and Aquatic Ecosystems, Adv. Sci., 12, 2406857, https://doi.org/10.1002/advs.202406857, 2025.
Hellemann, D., Tallberg, P., Bartl, I., Voss, M., and Hietanen, S.: Denitrification in an oligotrophic estuary: a delayed sink for riverine nitrate, Mar. Ecol. Prog. Ser., 583, 63–80, https://doi.org/10.3354/meps12359, 2017.
Hellemann, D., Tallberg, P., Aalto, S. L., Bartoli, M., and Hietanen, S.: Seasonal cycle of benthic denitrification and DNRA in the aphotic coastal zone, northern Baltic Sea, Mar. Ecol. Prog. Ser., 637, 15–28, https://doi.org/10.3354/meps13259, 2020.
Hietanen, S. and Kuparinen, J.: Seasonal and short-term variation in denitrification and anammox at a coastal station on the Gulf of Finland, Baltic Sea, Hydrobiologia, 596, 67–77, https://doi.org/10.1007/s10750-007-9058-5, 2008.
Hoffman, D. K., McCarthy, M. J., Newell, S. E., Gardner, W. S., Niewinski, D. N., Gao, J., and Mutchler, T. R.: Relative Contributions of DNRA and Denitrification to Nitrate Reduction in Thalassia testudinum Seagrass Beds in Coastal Florida (USA), Estuaries Coasts, 42, 1001–1014, https://doi.org/10.1007/s12237-019-00540-2, 2019.
Hou, E., Wen, D., Jiang, L., Luo, X., Kuang, Y., Lu, X., Chen, C., Allen, K. T., He, X., Huang, X., and Luo, Y.: Latitudinal patterns of terrestrial phosphorus limitation over the globe, Ecol. Lett., 24, 1420–1431, https://doi.org/10.1111/ele.13761, 2021.
Hsu, T.-C. and Kao, S.-J.: Technical Note: Simultaneous measurement of sedimentary N2 and N2O production and a modified 15N isotope pairing technique, Biogeosciences, 10, 7847–7862, https://doi.org/10.5194/bg-10-7847-2013, 2013.
Jäntti, H. and Hietanen, S.: The Effects of Hypoxia on Sediment Nitrogen Cycling in the Baltic Sea, AMBIO, 41, 161–169, https://doi.org/10.1007/s13280-011-0233-6, 2012.
Jäntti, H., Stange, F., Leskinen, E., and Hietanen, S.: Seasonal variation in nitrification and nitrate-reduction pathways in coastal sediments in the Gulf of Finland, Baltic Sea, Aquat. Microb. Ecol., 63, 171–181, https://doi.org/10.3354/ame01492, 2011.
Kennedy, C. D.: Nitrogen Overload: Environmental Degradation, Ramifications, and Economic Costs, Groundwater, 59, 161–162, https://doi.org/10.1111/gwat.13066, 2021.
Kessler, A. J., Roberts, K. L., Bissett, A., and Cook, P. L. M.: Biogeochemical Controls on the Relative Importance of Denitrification and Dissimilatory Nitrate Reduction to Ammonium in Estuaries, Global Biogeochem. Cy., 32, 1045–1057, https://doi.org/10.1029/2018GB005908, 2018.
Koop-Jakobsen, K. and Giblin, A. E.: The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments, Limnol. Oceanogr., 55, 789–802, https://doi.org/10.4319/lo.2010.55.2.0789, 2010.
Laffitte, B., Zhou, T., Yang, Z., Ciais, P., Jian, J., Huang, N., Seyler, B. C., Pei, X., and Tang, X.: Timescale Matters: Finer Temporal Resolution Influences Driver Contributions to Global Soil Respiration, Glob. Change Biol., 31, e70118, https://doi.org/10.1111/gcb.70118, 2025.
Li, N., Somes, C. J., Landolfi, A., Chien, C.-T., Pahlow, M., and Oschlies, A.: Global impact of benthic denitrification on marine N2 fixation and primary production simulated by a variable-stoichiometry Earth system model, Biogeosciences, 21, 4361–4380, https://doi.org/10.5194/bg-21-4361-2024, 2024.
Ling, J., Dungait, J. A. J., Delgado-Baquerizo, M., Cui, Z., Zhou, R., Zhang, W., Gao, Q., Chen, Y., Yue, S., Kuzyakov, Y., Zhang, F., Chen, X., and Tian, J.: Soil organic carbon thresholds control fertilizer effects on carbon accrual in croplands worldwide, Nat. Commun., 16, 3009, https://doi.org/10.1038/s41467-025-57981-6, 2025.
Liu, C., Hou, L., Liu, M., Zheng, Y., Yin, G., Han, P., Dong, H., Gao, J., Gao, D., Chang, Y., and Zhang, Z.: Coupling of denitrification and anaerobic ammonium oxidation with nitrification in sediments of the Yangtze Estuary: Importance and controlling factors, Estuarine, Coastal Shelf Sci., 220, 64–72, https://doi.org/10.1016/j.ecss.2019.02.043, 2019.
Liu, C., Hou, L., Liu, M., Zheng, Y., Yin, G., Dong, H., Liang, X., Li, X., Gao, D., and Zhang, Z.: In situ nitrogen removal processes in intertidal wetlands of the Yangtze Estuary, J. Environ. Sci., 93, 91–97, https://doi.org/10.1016/j.jes.2020.03.005, 2020.
Magri, M., Benelli, S., Bonaglia, S., Zilius, M., Castaldelli, G., and Bartoli, M.: The effects of hydrological extremes on denitrification, dissimilatory nitrate reduction to ammonium (DNRA) and mineralization in a coastal lagoon, Sci. Total Environ., 740, 140169, https://doi.org/10.1016/j.scitotenv.2020.140169, 2020.
McTigue, N. D., Gardner, W. S., Dunton, K. H., and Hardison, A. K.: Biotic and abiotic controls on co-occurring nitrogen cycling processes in shallow Arctic shelf sediments, Nat. Commun., 7, 13145, https://doi.org/10.1038/ncomms13145, 2016.
Meyer, R. L., Risgaard-Petersen, N., and Allen, D. E.: Correlation between Anammox Activity and Microscale Distribution of Nitrite in a Subtropical Mangrove Sediment, Appl. Environ. Microb., 71, 6142–6149, https://doi.org/10.1128/AEM.71.10.6142-6149.2005, 2005.
Middelburg, J. J., Soetaert, K., Herman, P. M. J., and Heip, C. H. R.: Denitrification in marine sediments: A model study, Global Biogeochem. Cy., 10, 661–673, https://doi.org/10.1029/96GB02562, 1996.
Na, T., Thamdrup, B., Kim, B., Kim, S.-H., Vandieken, V., Kang, D.-J., and Hyun, J.-H.: N2 production through denitrification and anammox across the continental margin (shelf–slope–rise) of the Ulleung Basin, East Sea, Limnol. Oceanogr., 63, S410–S424, https://doi.org/10.1002/lno.10750, 2018.
Neubacher, E. C., Parker, R. E., and Trimmer, M.: Short-term hypoxia alters the balance of the nitrogen cycle in coastal sediments, Limnol. Oceanogr., 56, 651–665, https://doi.org/10.4319/lo.2011.56.2.0651, 2011.
Nielsen, L. P.: Denitrification in sediment determined from nitrogen isotope pairing, FEMS Microbiol. Lett., 86, 357–362, https://doi.org/10.1016/0378-1097(92)90800-4, 1992.
Nielsen, L. P. and Glud, R. N.: Denitrification in a coastal sediment measured in situ by the nitrogen isotope pairing technique applied to a benthic flux chamber, Mar. Ecol. Prog. Ser., 137, 181–186, https://doi.org/10.3354/meps137181, 1996.
Poulin, P., Pelletier, E., and Saint-Louis, R.: Seasonal variability of denitrification efficiency in northern salt marshes: An example from the St. Lawrence Estuary, Mar. Environ. Res., 63, 490–505, https://doi.org/10.1016/j.marenvres.2006.12.003, 2007.
Richardson, K., Steffen, W., Lucht, W., Bendtsen, J., Cornell, S. E., Donges, J. F., Drüke, M., Fetzer, I., Bala, G., von Bloh, W., Feulner, G., Fiedler, S., Gerten, D., Gleeson, T., Hofmann, M., Huiskamp, W., Kummu, M., Mohan, C., Nogués-Bravo, D., Petri, S., Porkka, M., Rahmstorf, S., Schaphoff, S., Thonicke, K., Tobian, A., Virkki, V., Wang-Erlandsson, L., Weber, L., and Rockström, J.: Earth beyond six of nine planetary boundaries, Sci. Adv., 9, eadh2458, https://doi.org/10.1126/sciadv.adh2458, 2023.
Rios-Del Toro, E. E., Valenzuela, E. I., López-Lozano, N. E., Cortés-Martínez, M. G., Sánchez-Rodríguez, M. A., Calvario-Martínez, O., Sánchez-Carrillo, S., and Cervantes, F. J.: Anaerobic ammonium oxidation linked to sulfate and ferric iron reduction fuels nitrogen loss in marine sediments, Biodegradation, 29, 429–442, https://doi.org/10.1007/s10532-018-9839-8, 2018.
Risgaard-Petersen, N., Nielsen, L. P., Rysgaard, S., Dalsgaard, T., and Meyer, R. L.: Application of the isotope pairing technique in sediments where anammox and denitrification coexist, Limnol. Oceanogr. Meth., 1, 63–73, https://doi.org/10.4319/lom.2003.1.63, 2003.
Risgaard-Petersen, N., Meyer, R. L., Schmid, M., Jetten, M., S. M., Enrich-Prast, A., Rysgaard, S., and Revsbech, N. P.: Anaerobic ammonium oxidation in an estuarine sediment, Aquat. Microb. Ecol., 36, 293–304, https://doi.org/10.3354/ame036293, 2004.
Robertson, E. K., Bartoli, M., Brüchert, V., Dalsgaard, T., Hall, P. O. J., Hellemann, D., Hietanen, S., Zilius, M., and Conley, D. J.: Application of the isotope pairing technique in sediments: Use, challenges, and new directions, Limnol. Oceanogr. Meth., 17, 112–136, https://doi.org/10.1002/lom3.10303, 2019.
Rosales Villa, A. R., Jickells, T. D., Sivyer, D. B., Parker, E. R., and Thamdrup, B.: Benthic nitrogen cycling in the North Sea, Cont. Shelf Res., 185, 31–36, https://doi.org/10.1016/j.csr.2018.05.005, 2019.
Rysgaard, S., Finster, K., and Dahlgaard, H.: Primary production, nutrient dynamics and mineralisation in a northeastern Greenland fjord during the summer thaw, Polar Biol., 16, 497–506, https://doi.org/10.1007/BF02329069, 1996a.
Rysgaard, S., Risgaard-Petersen, N., and Sloth, N. P.: Nitrification, denitrification, and nitrate ammonification in sediments of two coastal lagoons in Southern France, Hydrobiologia, 329, 133–141, https://doi.org/10.1007/BF00034553, 1996b.
Rysgaard, S., Fossing, H., and Jensen, M. M.: Organic matter degradation through oxygen respiration, denitrification, and manganese, iron, and sulfate reduction in marine sediments (the Kattegat and the Skagerrak), Ophelia, 55, 77–91, https://doi.org/10.1080/00785236.2001.10409475, 2001.
Rysgaard, S., Glud, R. N., Risgaard-Petersen, N., and Dalsgaard, T.: Denitrification and anammox activity in Arctic marine sediments, Limnol. Oceanogr., 49, 1493–1502, https://doi.org/10.4319/lo.2004.49.5.1493, 2004.
Salk, K. R., Erler, D. V., Eyre, B. D., Carlson-Perret, N., and Ostrom, N. E.: Unexpectedly high degree of anammox and DNRA in seagrass sediments: Description and application of a revised isotope pairing technique, Geochim. Cosmochim. Ac., 211, 64–78, https://doi.org/10.1016/j.gca.2017.05.012, 2017.
Samperio-Ramos, G., Hernández-Sánchez, O., Camacho-Ibar, V. F., Pajares, S., Gutiérrez, A., Sandoval-Gil, J. M., Reyes, M., De Gyves, S., Balint, S., Oczkowski, A., Ponce-Jahen, S. J., and Cervantes, F. J.: Ammonium loss microbiologically mediated by Fe(III) and Mn(IV) reduction along a coastal lagoon system, Chemosphere, 349, 140933, https://doi.org/10.1016/j.chemosphere.2023.140933, 2024.
Sokoll, S., Holtappels, M., Lam, P., Collins, G., Schlüter, M., Lavik, G., and Kuypers, M.: Benthic Nitrogen Loss in the Arabian Sea Off Pakistan, Front. Microbiol., 3, 395, https://doi.org/10.3389/fmicb.2012.00395, 2012.
Song, G., Liu, S., Zhu, Z., Zhai, W., Zhu, C., and Zhang, J.: Sediment oxygen consumption and benthic organic carbon mineralization on the continental shelves of the East China Sea and the Yellow Sea, Deep Sea-Res. Pt. II, 124, 53–63, https://doi.org/10.1016/j.dsr2.2015.04.012, 2016a.
Song, G., Liu, S., Zhang, J., Zhu, Z., Zhang, G., Marchant, H. K., Kuypers, M. M. M., and Lavik, G.: Response of benthic nitrogen cycling to estuarine hypoxia, Limnol. Oceanogr., 66, 652–666, https://doi.org/10.1002/lno.11630, 2021.
Song, G. D., Liu, S. M., Kuypers, M. M. M., and Lavik, G.: Application of the isotope pairing technique in sediments where anammox, denitrification, and dissimilatory nitrate reduction to ammonium coexist, Limnol. Oceanogr. Meth., 14, 801–815, https://doi.org/10.1002/lom3.10127, 2016b.
Steingruber, S. M., Friedrich, J., Gächter, R., and Wehrli, B.: Measurement of Denitrification in Sediments with the 15N Isotope Pairing Technique, Appl. Environ. Microb., 67, 3771–3778, https://doi.org/10.1128/AEM.67.9.3771-3778.2001, 2001.
Strous, M., Fuerst, J. A., Kramer, E. H. M., Logemann, S., Muyzer, G., van de Pas-Schoonen, K. T., Webb, R., Kuenen, J. G., and Jetten, M. S. M.: Missing lithotroph identified as new planctomycete, Nature, 400, 446–449, https://doi.org/10.1038/22749, 1999.
Susanna, H.: Anaerobic ammonium oxidation (anammox) in sediments of the Gulf of Finland, Aquat. Microb. Ecol., 48, 197–205, https://doi.org/10.3354/ame048197, 2007.
Tan, E., Zou, W., Jiang, X., Wan, X., Hsu, T.-C., Zheng, Z., Chen, L., Xu, M., Dai, M., and Kao, S.-J.: Organic matter decomposition sustains sedimentary nitrogen loss in the Pearl River Estuary, China, Sci. Total Environ., 648, 508–517, https://doi.org/10.1016/j.scitotenv.2018.08.109, 2019.
Tan, E., Zou, W., Zheng, Z., Yan, X., Du, M., Hsu, T.-C., Tian, L., Middelburg, J. J., Trull, T. W., and Kao, S.-J.: Warming stimulates sediment denitrification at the expense of anaerobic ammonium oxidation, Nat. Clim. Change, 10, 349–355, https://doi.org/10.1038/s41558-020-0723-2, 2020.
Tan, E., Hsu, T.-C., Zou, W., Yan, X., Huang, Z., Chen, B., Chang, Y., Zheng, Z., Zheng, L., Xu, M., Tian, L., and Kao, S.-J.: Quantitatively deciphering the roles of sediment nitrogen removal in environmental and climatic feedbacks in two subtropical estuaries, Water Res., 224, 119121, https://doi.org/10.1016/j.watres.2022.119121, 2022.
Thamdrup, B.: New Pathways and Processes in the Global Nitrogen Cycle, Annu. Rev. Ecol. Evol. S., 43, 407–428, https://doi.org/10.1146/annurev-ecolsys-102710-145048, 2012.
Thamdrup, B. and Dalsgaard, T.: Production of N2 through Anaerobic Ammonium Oxidation Coupled to Nitrate Reduction in Marine Sediments, Appl. Environ. Microb., 68, 1312–1318, https://doi.org/10.1128/AEM.68.3.1312-1318.2002, 2002.
Torregrosa-Crespo, J., Miralles-Robledillo, J. M., Bernabeu, E., Pire, C., and Martínez-Espinosa, R. M.: Denitrification in hypersaline and coastal environments, FEMS Microbiol. Lett., 370, fnad066, https://doi.org/10.1093/femsle/fnad066, 2023.
Trimmer, M. and Nicholls, J. C.: Production of nitrogen gas via anammox and denitrification in intact sediment cores along a continental shelf to slope transect in the North Atlantic, Limnol. Oceanogr., 54, 577–589, https://doi.org/10.4319/lo.2009.54.2.0577, 2009.
Trimmer, M., Engström, P., and Thamdrup, B.: Stark Contrast in Denitrification and Anammox across the Deep Norwegian Trench in the Skagerrak, Appl. Environ. Microb., 79, 7381–7389, https://doi.org/10.1128/AEM.01970-13, 2013.
Trimmer, M., Nicholls, J. C., and Deflandre, B.: Anaerobic Ammonium Oxidation Measured in Sediments along the Thames Estuary, United Kingdom, Appl. Environ. Microb., 69, 6447–6454, https://doi.org/10.1128/AEM.69.11.6447-6454.2003, 2003.
Trimmer, M., Risgaard-Petersen, N., Nicholls, J. C., and Engström, P.: Direct measurement of anaerobic ammonium oxidation (anammox) and denitrification in intact sediment cores, Mar. Ecol. Prog. Ser., 326, 37–47, https://doi.org/10.3354/meps326037, 2006.
Usui, T., Koike, I., and Ogura, N.: N2O Production, Nitrification and Denitrification in an Estuarine Sediment, Estuarine, Coastal Shelf Sci., 52, 769–781, https://doi.org/10.1006/ecss.2000.0765, 2001.
Uusheimo, S., Huotari, J., Tulonen, T., Aalto, S. L., Rissanen, A. J., and Arvola, L.: High Nitrogen Removal in a Constructed Wetland Receiving Treated Wastewater in a Cold Climate, Environ. Sci. Technol., 52, 13343–13350, https://doi.org/10.1021/acs.est.8b03032, 2018.
Vance-Harris, C. and Ingall, E.: Denitrification pathways and rates in the sandy sediments of the Georgia continental shelf, USA, Geochem. T., 6, 12, https://doi.org/10.1186/1467-4866-6-12, 2005.
van de Graaf, A. A., Mulder, A., de Bruijn, P., Jetten, M. S., Robertson, L. A., and Kuenen, J. G.: Anaerobic oxidation of ammonium is a biologically mediated process, Appl. Environ. Microb., 61, 1246–1251, https://doi.org/10.1128/aem.61.4.1246-1251.1995, 1995.
Wan, R., Ge, L., Chen, B., Tang, J.-M., Tan, E., Zou, W., Tian, L., Li, M., Liu, Z., Hou, L., Yin, G., and Kao, S.-J.: Permeability decides the effect of antibiotics on sedimentary nitrogen removal in Jiulong River Estuary, Water Res., 243, 120400, https://doi.org/10.1016/j.watres.2023.120400, 2023.
Welsh, D. T., Bartoli, M., Nizzoli, D., Castaldelli, G., Riou, S. A., and Viaroli, P.: Denitrification, nitrogen fixation, community primary productivity and inorganic-N and oxygen fluxes in an intertidal Zostera noltii meadow, Mar. Ecol. Prog. Ser., 208, 65–77, https://doi.org/10.3354/meps208065, 2000.
Yang, J.-Y. T., Hsu, T.-C., Tan, E., Lee, K., Krom, M. D., Kang, S., Dai, M., Hsiao, S. S.-Y., Yan, X., Zou, W., Tian, L., and Kao, S.-J.: Sedimentary processes dominate nitrous oxide production and emission in the hypoxic zone off the Changjiang River estuary, Sci. Total Environ., 827, 154042, https://doi.org/10.1016/j.scitotenv.2022.154042, 2022.
Yin, G., Hou, L., Zong, H., Ding, P., Liu, M., Zhang, S., Cheng, X., and Zhou, J.: Denitrification and Anaerobic Ammonium Oxidization Across the Sediment–Water Interface in the Hypereutrophic Ecosystem, Jinpu Bay, in the Northeastern Coast of China, Estuaries Coasts, 38, 211–219, https://doi.org/10.1007/s12237-014-9798-1, 2015.