the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Long-term monitoring of hydrological dynamics and phytoplankton biomass indicator in three shellfish ecosystems of the English channel (2000–2024)
Stéphanie Petinay
Jean-Louis Blin
This data paper presents a harmonized and quality-controlled dataset resulting from 24 years (2000–2024) of monthly monitoring at three coastal stations in Normandy, France: Blainville-sur-Mer, Saint-Vaast-la-Hougue, and Utah Beach. The dataset includes measurements of key physico-chemical parameters (temperature, salinity, pH, dissolved oxygen), biogeochemical variables (dissolved nutrients: nitrate, ammonium, phosphate, and silicate), and biological indicators (chlorophyll a concentration). Sampling was conducted using consistent protocols across stations, allowing for long-term comparison. All data have undergone validation and standardization to ensure their usability for environmental assessment and modeling purposes. The dataset provides valuable insights into the evolution of coastal water quality under the influence of climate change and anthropogenic pressures. Notable trends include increasing winter temperatures (especially since 2010), progressive ocean acidification at Blainville-sur-Mer, and changing nutrient regimes across the region, with a marked phosphate decline and spatial contrasts in nutrient limitation (phosphorus-limited on the East coast, nitrogen-limited on the West coast). These long-term observations are critical for understanding ecosystem dynamics, supporting coastal management strategies, and informing sustainable aquaculture development. The dataset is openly available and intended to support further interdisciplinary research in marine science and environmental policy (SMEL, 2024, https://doi.org/10.5281/zenodo.15058835).
- Article
(6684 KB) - Full-text XML
- BibTeX
- EndNote
Located at the interface between land and ocean, coastal ecosystems are highly productive areas essential for the survival of many marine species (Barbier et al., 2011). However, these environments are under increasing pressure from human activities, such as eutrophication, pollution, and changes to coastal and marine landscapes. These threats affect both benthic and pelagic habitats, thereby disrupting ecological balances (Sala et al., 2000; Dudgeon et al., 2006; Halpern et al., 2007; Barbier et al., 2011; Ovaskainen et al., 2019).
Since the 1950s, the widespread use of fertilizers in intensive agricultural practices has led to an increase in nutrient inputs into European coastal waters (Vermaat et al., 2008). Over the decades, numerous programs have been implemented to limit these discharges, but while the effects on phosphorus inputs have been notable (Claussen et al., 2009), nitrogen inputs remain very high (Garnier et al., 2019). These inputs influence not only the concentration of nutrients but also their stoichiometry (Martin et al., 2008; Watanabe et al., 2017; Meybeck et al., 2018). These imbalances lead to changes in the productivity of phytoplankton communities, which are at the base of the food web, as well as in their composition (Shen, 2001; Cadée and Hegeman, 2002; Smith, 2006; Lefebvre et al., 2011; Leruste et al., 2019).
Nutrient inputs, alongside other environmental factors such as light availability, temperature, water residence time, and river discharge, play a critical role in driving phytoplankton blooms (Heisler et al., 2008). These blooms are vital for shellfish ecosystems, serving as an essential food source for farmed bivalves (Sonier et al., 2016; Filgueira et al., 2016). However, studies have reported a slowdown in primary production, including phytoplankton blooms, due to changing environmental conditions (Romero et al., 2016).
Climate change significantly influences coastal systems through various physical and chemical processes (Kirby et al., 2009). Beyond the well-documented direct effects of rising temperatures on marine organisms (Beaugrand, 2004), warming also affects water stratification, which alters the vertical exchange of nutrients and dissolved oxygen (Sarmiento and Gruber, 2006). This can lead to an increased occurrence of hypoxic or anoxic events in coastal waters (Diaz, 2001; Selman et al., 2008). Furthermore, atmospheric circulation – through changes in sea level pressure, wind direction, and intensity-impacts oceanic currents, which play a key role in the horizontal transport of nutrients and dissolved oxygen in these ecosystems (Cloern, 2001; Reid et al., 2003).
Since the early 2000s, the HYDRONOR observatory has been dedicated to monitoring the water quality of shellfish farming areas in the Cotentin Peninsula (Normandy). This observatory provides one of the longest and most comprehensive time series on hydrobiological parameters available for this region. The objective of the present study is to offer a robust dataset of key environmental variables at three representative shellfish stations – Blainville-sur-Mer, Saint-Vaast-La-Hougue, and Utah Beach – monitored by HYDRONOR. Over 20 years of high-frequency data have been compiled, offering robust insights into coastal dynamics in response to anthropogenic pressures and climate variability.
This dataset is particularly valuable due to its regional scale, high temporal resolution, and methodological consistency. It complements national initiatives such as the SOMLIT program (Service d'Observation en Milieu Littoral), which monitors long-term trends in coastal waters at key research sites across France. While SOMLIT stations such as Luc-sur-Mer (Baie de Seine) and Roscoff provide critical data on large-scale trends, HYDRONOR offers a higher spatial resolution within a single department with a focus on shellfish-farming ecosystems. As such, it bridges the gap between broader-scale observations and local aquaculture management needs.
2.1 Sampling sites and strategies
Among the coastal stations monitored by the HYDRONOR observatory, three were selected for this dataset: Blainville-sur-Mer, Saint-Vaast-la-Hougue, and Utah Beach. These stations were chosen primarily because they offer the most complete and continuous datasets since the beginning of the monitoring program in 2000, ensuring the robustness and reliability of long-term analyses. In addition to their data quality, these sites represent contrasting environmental conditions that allow for the exploration of a representative gradient along the Normandy coastline. Two of these areas are located in open ecosystems, heavily exploited by oyster farming activities: Saint-Vaast-la-Hougue, in the eastern part of the department, and Blainville-sur-Mer, in the western part. The third area, Utah Beach, is located in the enclosed environment of the Baie des Veys, under strong anthropogenic influence (Fig. 1). These include intensive agricultural activity in the watershed (particularly livestock farming and maize cultivation), leading to diffuse nutrient inputs via river discharges (notably from the Douve, Taute, and Vire rivers) (Grangere, 2004; Chenel, 2025).
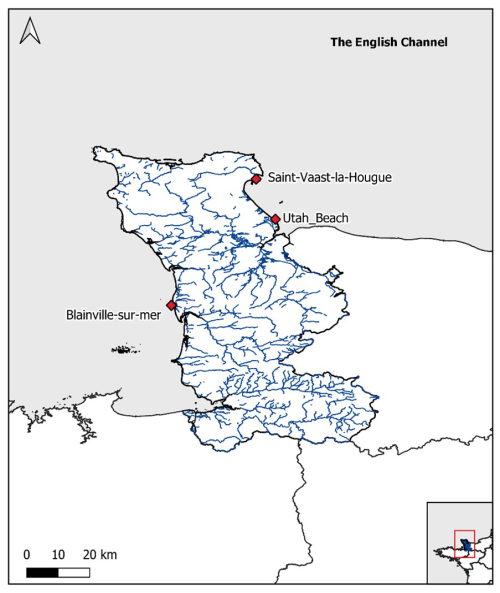
Figure 1Location of the sampling stations along the French coast in the English Channel (Blainville-sur-mer, Saint-Vaast-La-Hougue, Utah Beach). The blue lines represent the river network of the department.
The analysis focused on key environmental variables directly involved in the stoichiometry of primary production: dissolved inorganic nitrogen (DIN = NO + NO + NH), orthophosphate (PO), and reactive silicate (Si(OH)4), as well as chlorophyll a, used as a proxy for phytoplankton biomass. These variables were selected due to their ecological relevance and the consistency of their measurement across the full study period and all three stations.
2.2 Sample analysis
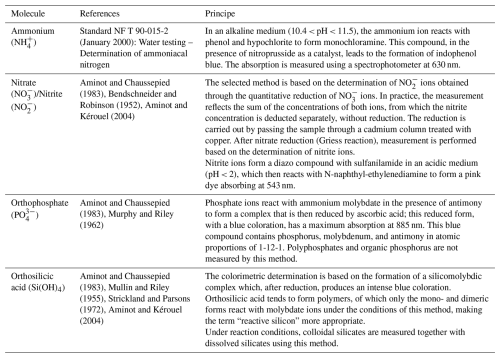
The samples were collected at a depth of 1 m using a 5 L Niskin bottle, twice a month, one hour before or after high tide. Chlorophyll a concentrations were determined following filtration of three 100 mL aliquots through glass fiber filters (Whatman GF/F, nominal pore size 0.7 µm). Filters were stored frozen (−20 °C) and extracted in 90 % acetone for 12–24 h in the dark at 4 °C. Measurements were performed using a Turner Designs fluorometer, following the method described by Strickland and Parsons (1972). Results are expressed in µg L−1. Nutrients were measured via spectrophotometry. Mineral nitrogen was quantified through ammonium (NH), nitrite (NO), and nitrate (NO). Only the assimilable form of phosphorus, orthophosphate (PO), was quantified, as well as silicates (Si(OH)4). The methods are detailed in Table 1.
Nutrient samples were pre-filtered at the time of collection using a 50 µm mesh filter mounted directly on the Niskin bottle to remove large particulate matter. After collection, all samples were centrifuged for 10 min at 4200 rpm to eliminate residual suspended particles. This approach ensures that only the dissolved fraction is analyzed. Samples were then stored at 4 °C in the dark and analyzed within 4 h after sampling to minimize any transformation or degradation of the compounds.
Calibration standards were prepared using low-nutrient seawater and validated at the SMEL laboratory (Synergie Mer and Littoral, France), in accordance with the French standard AFNOR XP T90-210 (May 2009), which defines the protocol for the initial performance evaluation of analytical methods in environmental laboratories.
In situ temperature and salinity were recorded using a YSI 6600 multi-parameter probe, calibrated prior to each field campaign according to the manufacturer's instructions. Salinity calibration was performed using certified NaCl standards (35 PSU). The accuracy of the salinity sensor was ±0.1 PSU, and for temperature ± 0.15 °C. The probe was systematically verified against atmospheric pressure measurements to ensure the reliability of barometric compensation, particularly for sensors sensitive to pressure fluctuations (e.g. pH or oxygen, if applicable). The pH was measured in the laboratory using a Mettler Toledo F2 pH-meter equipped with a LE420 glass electrode. The instrument was calibrated daily using standard buffer solutions (pH 4.00, 7.00, and 10.00 at 20 °C). Measurements were performed at a controlled temperature of 20 °C, on the NBS scale, without CO2 equilibration. Water samples were stored in the dark at 4 °C and analyzed within 4 h after collection to limit any biological or chemical alterations. To assess the potential limitation of primary production by nutrient availability, the standard molar ratios for dissolved inorganic nitrogen (DIN = ammonium + nitrite + nitrate), phosphate, and silicate were calculated and compared. These ratios were based on the biogenic matter composition described by Redfield et al. (1963) and Brzezinski (1985), which is Si : N : P = 16 : 16 : 1.
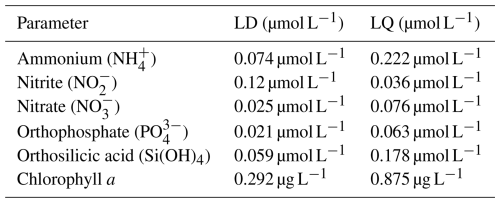
Additionally, to evaluate the analytical sensitivity and ensure proper data interpretation, the limits of detection (LD) and limits of quantification (LQ) were determined for each parameter based on method validation procedures carried out in accordance with the AFNOR XP T90-210 standard. These values are summarized in Table 2.
2.3 Data analysis
The entire dataset was analyzed using the R statistical software (R Core Team, 2022), with the TTAinterfaceTrendAnalysis package developed by Devreker and Lefebvre (2014). This package is specifically designed for the analysis of long-term environmental time series. It applies non-parametric statistical methods such as the Seasonal Kendall test and Sen's slope estimator, which are robust to non-normal data distributions, missing values, and outliers – characteristics commonly encountered in environmental datasets. The package enables the detection and quantification of monotonic trends, as well as the evaluation of seasonal and interannual variability. This makes it particularly suitable for identifying long-term changes in parameters such as temperature, pH, and nutrient concentrations in coastal ecosystems.
3.1 Evolution of physico-chemical parameters: increase in winter temperature and pH
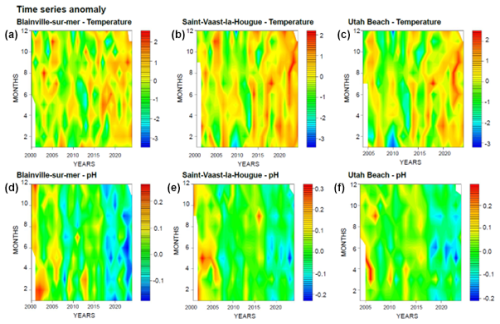
Figure 2 presents the monitoring of physico-chemical parameters. The monthly trends across the three stations show a similar pattern regarding temperature. However, the time series reveals a notable increase in winter temperatures. For instance, between 2009 and 2013, the average minimum temperatures at the three stations were around 5 °C. In contrast, from 2013 to 2024, these minimum temperatures ranged between 6 and 8 °C across all stations.
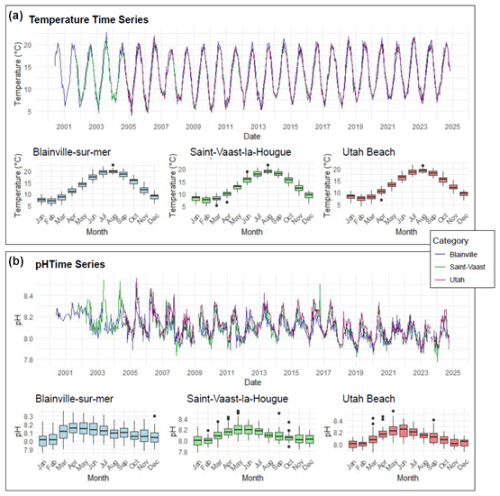
Figure 2Time Series and Monthly Averages of Temperature (°C) (a) and pH (b) from 2000 to 2024 at Blainville-sur-Mer, Saint-Vaast-la-Hougue, and Utah Beach. Boxplots show the 25th–75th percentile range (interquartile range, IQR), with the median as a horizontal line. Whiskers extend to 1.5 × IQR; points beyond are outliers.
Changes are also observed in seawater pH, which has gradually declined over the monitoring period (Fig. 2). In the early 2000s, the lowest recorded pH remained above 8.0, whereas by 2024, it had decreased to 7.85. These trends over time are further illustrated by the anomaly plots presented in Fig. 3. The red anomalies for temperature represent values above the interannual averages (positive anomalies) and show an increasing frequency and intensity over time, particularly in recent years. For pH, the dominance of blue anomalies since 2017 indicates a progressive acidification, most noticeable at Blainville-sur-Mer, on the western coast, but also evident at the other two sites.
3.2 Spatial and temporal variability of chlorophyll a concentrations
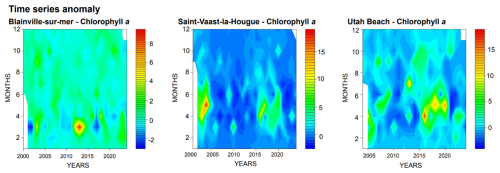
Chlorophyll a concentrations are illustrated in Fig. 4. The stations located on the eastern coast, Saint-Vaast-la-Hougue and Utah Beach, exhibit the highest levels, with peaks exceeding 20 µg L−1, compared to a maximum of 10 µg L−1 at Blainville-sur-Mer. On the western coast, the annual bloom is clearly visible, marked by an increase in chlorophyll a concentrations every March.
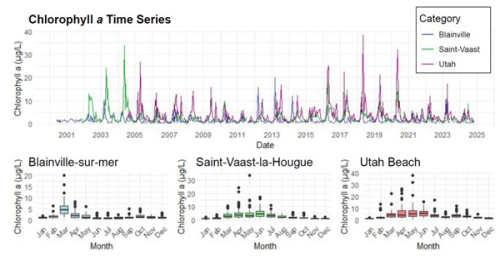
Figure 4Time Series and Monthly Averages of chlorophyll a (µg L−1) from 2000 to 2024 at Blainville-sur-Mer, Saint-Vaast-la-Hougue (Tocquaise), and Utah Beach.
Since 2020, a drastic decrease in chlorophyll a concentrations has been observed on the eastern coast. This trend is confirmed by Fig. 5, which highlights values below the median for this period. Moreover, the predominance of green in the Blainville graph, compared to blue for the other two stations, underscores the differences between the western and eastern coastal ecosystems.
3.3 Spatial and temporal variability of inorganic nutrient concentration
The evolution and trends of dissolved nutrients are illustrated in Fig. 6. Ammonium shows the most marked variations between the stations, with higher concentrations observed on the East coast (Saint-Vaast-la-Hougue), where values can exceed 2 µmol L−1, compared to the West coast (Blainville-sur-Mer), where maximum concentrations are closer to 1 µmol L−1. Ammonium levels tend to increase between September and December at all sites. Nitrites and nitrates exhibit more cyclical and synchronized seasonal variations across the three stations. Their concentrations are lowest in summer, then increase significantly during the winter, peaking in January at values exceeding 20 µmol L−1, before gradually decreasing in the spring. On the West coast, although the maximum concentrations are similar to those of the East coast, they decrease more rapidly once the winter peak is reached. As for orthophosphates and silicates, the trends follow a similar pattern to those of nitrates plus nitrites: concentrations rise in the autumn, peak in the winter, and decrease in the spring. For phosphate, concentrations rarely exceed 1 µmol L−1, while silicates reach nearly 30 µmol L−1 in winter, especially on the East coast. However, no difference was observed between the two coasts for silicates. Finally, the time series for phosphate highlights a gradual decline in concentrations over the past 15 years on both coasts.
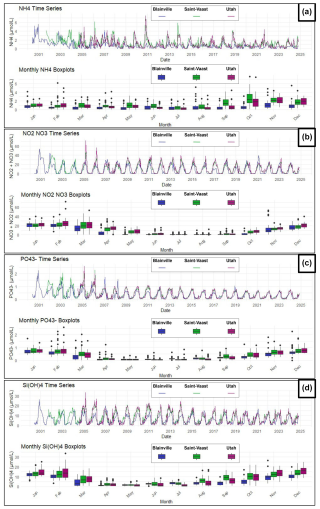
Figure 6Time series and monthly averages of nutrient concentrations at the three stations (2000–2024): Blainville-sur-Mer, Saint-Vaast-la-Hougue, and Utah Beach. (a) Ammonium (NH), (b) Nitrites and Nitrates (NO, NO), (c) Orthophosphate (PO), (d) Orthosilicic acid (Si(OH)4).
Figure 7 shows the seasonal decomposition of chlorophyll a concentrations. A well-defined seasonal cycle is visible at each station, with spring peaks and summer declines. The long-term trend component reveals a gradual decrease in chlorophyll-a until around 2010, followed by a transient increase until 2015, and then a continued decline. Over the full 2000–2024 period, total chlorophyll-a concentrations decreased by 0.2 µg L−1 at Blainville-sur-Mer, 3.0 µg L−1 at Saint-Vaast-la-Hougue, and 2.0 µg L−1 at Utah Beach. The remainder components show moderate interannual variability.
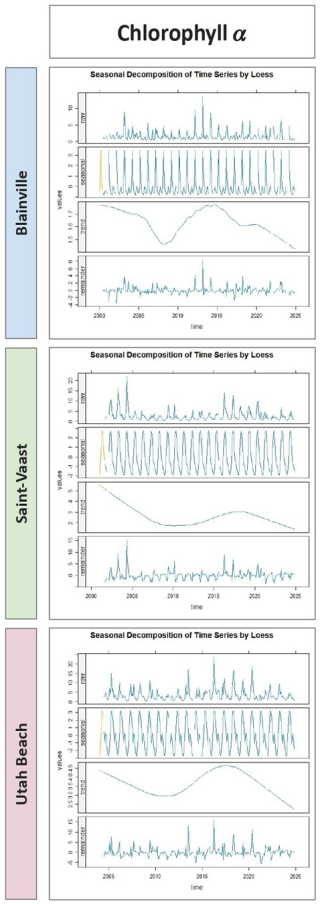
Figure 7Seasonal Decomposition of Chlorophyll a Concentrations (µg L−1) from 2000 to 2024 at Blainville-sur-Mer, Saint-Vaast-la-Hougue, and Utah Beach. For each station, four panels are shown: the original time series (Raw), the seasonal component (Seasonal), the long-term trend (Trend), and the residuals (Remainder). The decomposition was performed using LOESS smoothing with the TrendAnalysis function from the TTAinterfaceTrendAnalysis R package.
The seasonal decomposition of nitrogenous nutrients (ammonium, nitrite, and nitrate) are represented in the Fig. 8. For nitrate and nitrite, a strong and consistent seasonal pattern is observed at all three stations, with pronounced winter maxima and summer minima. The seasonal signal remains stable over time, while the long-term trend indicates a general decline across all sites. Between 2000 and 2024, nitrate and nitrite concentrations decreased by approximately 6.0 µmol L−1 at Blainville-sur-Mer and Saint-Vaast-la-Hougue, and by 4.0 µmol L−1 at Utah Beach. Ammonium also shows a seasonal signal, although less regular and with more interannual variability. Its long-term trend indicates a decrease of about 1.5 µmol L−1 at Blainville-sur-Mer, 1.0 µmol L−1 at Saint-Vaast-la-Hougue, and 0.4 µmol L−1 at Utah Beach. The remainder component for ammonium is more variable than for nitrate and nitrite, suggesting a greater influence of short-term events.
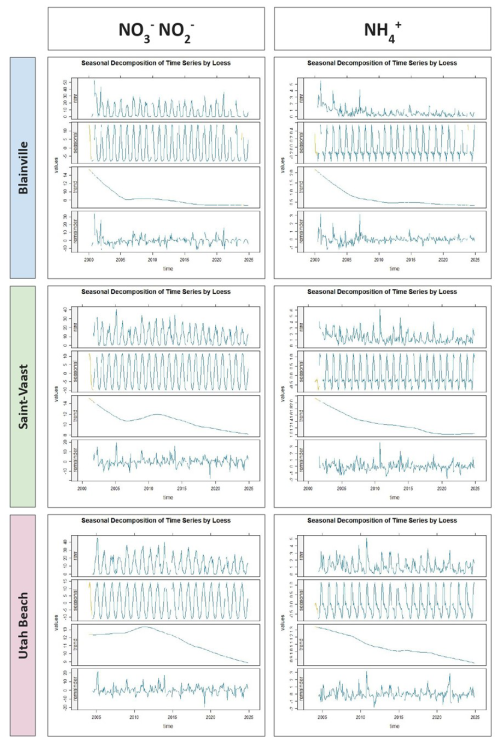
Figure 8Seasonal decomposition of dissolved inorganic nitrogen (ammonium, nitrate, and nitrite; µmol L−1) from 2000 to 2024 at the three sampling stations: Blainville-sur-Mer (blue), Saint-Vaast-la-Hougue (green), and Utah Beach (purple). For each parameter and station, four panels are shown: the original time series (Raw), the seasonal component (Seasonal), the long-term trend (Trend), and the residuals (Remainder). The decomposition was performed using LOESS smoothing with the TrendAnalysis function from the TTAinterfaceTrendAnalysis R package.
The seasonal decomposition of orthophosphates and silicates (Fig. 9) reveals a clear and recurrent annual cycle at all stations, characterized by peak concentrations in winter and minimum levels in summer. This seasonal component is remarkably stable across years. For phosphate, the trend shows a long-term decrease over the study period, with a reduction of approximately 0.3 µmol L−1 at Blainville-sur-Mer, 0.5 µmol L−1 at Saint-Vaast-la-Hougue, and 0.4 µmol L−1 at Utah Beach. Silicate concentrations also declined between 2000 and 2024, with reductions of 1.5 µmol L−1 at Blainville-sur-Mer, 2.0 µmol L−1 at Saint-Vaast-la-Hougue, and 2.5 µmol L−1 at Utah Beach. The residual components for phosphate and silicate show limited short-term fluctuations, indicating the predominance of seasonal and long-term components.
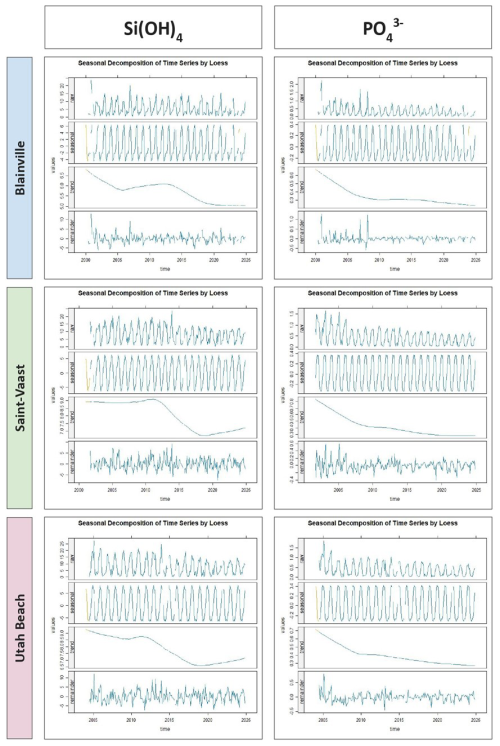
Figure 9Seasonal Decomposition of Orthophosphate and Orthosilicic acid (µmol L−1) from 2000 to 2024 at Blainville-sur-Mer, Saint-Vaast-la-Hougue, and Utah Beach. For each parameter and station, four panels are shown: the original time series (Raw), the seasonal component (Seasonal), the long-term trend (Trend), and the residuals (Remainder). The decomposition was performed using LOESS smoothing with the TrendAnalysis function from the TTAinterfaceTrendAnalysis R package.
Finally, although chlorophyll-a concentrations reached a minimum around 2010, this period did not coincide with minimum nutrient concentrations, suggesting asynchronous temporal dynamics.
3.4 Seasonal patterns in environmental data revealed by principal component analyses
At Blainville-sur-Mer (Fig. 10), the first principal component (Dim1) explains 49.2 % of the total variance, while the second component (Dim2) accounts for 16 %, resulting in a cumulative variance of 65.2 %. Vectors pointing to the right, which are strongly and positively correlated with Dim1, correspond to nutrient concentrations: nitrate, nitrite, phosphate, and silicate. These variables therefore explain a large part of the variability observed along Dim1. In contrast, vectors associated with dissolved oxygen, pH, and chlorophyll a point in the opposite direction, indicating a negative correlation with Dim1. Winter samples (in blue) are mostly located in the upper right quadrant of the plot, indicating an association with higher values of variables positively correlated with Dim1, such as nutrients. Conversely, spring samples (in green) tend to cluster in the lower left quadrant, suggesting an association with higher levels of dissolved oxygen (O2), pH, and chlorophyll a (Chlo a), which are negatively correlated with Dim1. Autumn (orange) and summer (yellow) samples are more concentrated around the origin, with less dispersion. Some sampling dates appear as outliers (e.g., March 2012, November 2000, March 2013), positioned at a distance from the main cluster of points.
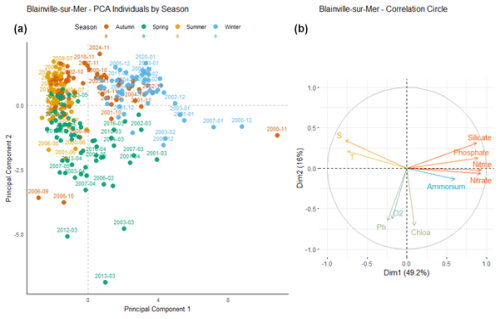
Figure 10Principal Component Analysis of seasonal variability in physicochemical parameters and dissolved nutrients at Blainville-sur-Mer. (a) Projection of sampling dates (2000–2024) on the first two principal components. Samples are colored by season: blue (winter), green (spring), yellow (summer), and orange (autumn). (b) Correlation circle showing the contribution of environmental variables: nutrients (nitrate, nitrite, ammonium, phosphate, silicate), dissolved oxygen (O2), pH, chlorophyll a (Chlo a), temperature (T), salinity (S).
At Saint-Vaast-la-Hougue (Fig. 11), the first principal component (Dim1) explains 46.6 % of the total variance, while the second component (Dim2) accounts for 21.4 %. Dim1 is positively correlated with nutrient concentrations, except for ammonium, whose vector is shorter, indicating a weaker contribution to this axis.
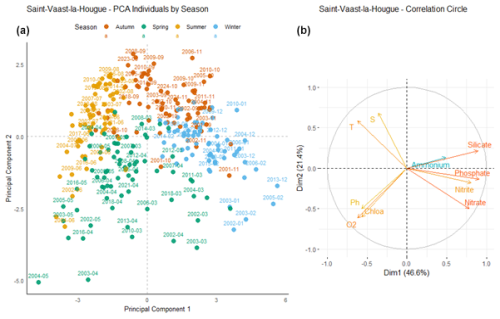
Figure 11Principal Component Analysis of seasonal variability in physicochemical parameters and dissolved nutrients at Saint-Vaast-la-Hougue. (a) Projection of sampling dates (2000–2024) on the first two principal components. Samples are colored by season: blue (winter), green (spring), yellow (summer), and orange (autumn). (b) Correlation circle showing the contribution of environmental variables: nutrients (nitrate, nitrite, ammonium, phosphate, silicate), dissolved oxygen (O2), pH, chlorophyll a (Chlo a), temperature (T), salinity (S).
The different seasons, represented by four distinct colors, form well-defined clusters. Blue points (winter months) are positively correlated with Dim1, whereas yellow points (summer months) are negatively correlated with this component. Green points (spring months) are located in the area associated with positive correlations to chlorophyll a and dissolved oxygen. Some overlap between seasons is observed, although seasonal clustering remains generally well structured.
At Utah Beach (Fig. 12), the first principal component (Dim1) explains 54.5 % of the total variance. It is positively correlated with nutrient concentrations such as nitrate, nitrite, phosphate, and silicate. Ammonium, shown in blue in the correlation circle, exhibits a weaker correlation with this component. Temperature, salinity, dissolved oxygen, and chlorophyll a are negatively correlated with Dim1. The second component (Dim2) accounts for 17.4 % of the variance.
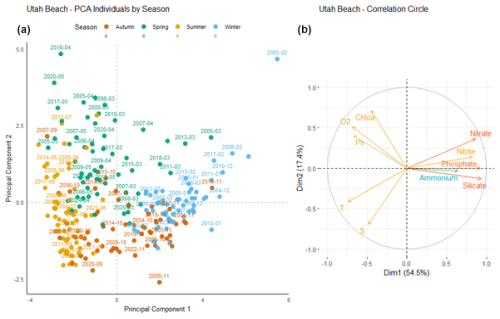
Figure 12Principal Component Analysis of seasonal variability in physicochemical parameters and dissolved nutrients at Utah Beach. (a) Projection of sampling dates (2000–2024) on the first two principal components. Samples are colored by season: blue (winter), green (spring), yellow (summer), and orange (autumn). (b) Correlation circle showing the contribution of environmental variables: nutrients (nitrate, nitrite, ammonium, phosphate, silicate), dissolved oxygen (O2), pH, chlorophyll a (Chlo a), temperature (T), salinity (S).
Winter observations (blue) are primarily located on the right-hand side of Dim1, associated with positive values and higher nutrient concentrations. In contrast, summer observations (yellow) tend to cluster on the left-hand side, where nutrient levels are lower. Spring samples (green) occupy an intermediate position, with some overlap, in the area associated with chlorophyll a and dissolved oxygen.
3.5 Stoichiometric Limitations
The variations in macronutrient concentrations described in Fig. 6 influenced the stoichiometric ratios.
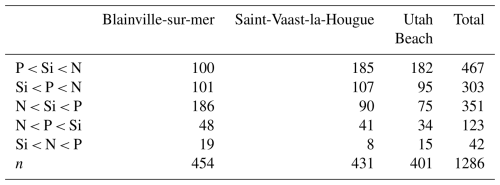
Data aggregated from 2000 to 2024 reveal two distinct nutrient limitation dynamics depending on the coastline. At Blainville-sur-Mer, nitrogen was identified as the primary limiting nutrient in 186 observations, followed by silica (101 observations), while phosphorus was least frequently limiting (100 observations) (Table 3). On the eastern coast, phosphorus appeared most frequently as the limiting nutrient, with 185 observations at Saint-Vaast-la-Hougue and 182 at Utah Beach. In both of these stations, silica and nitrogen were less frequently limiting, with 107 and 95 observations for silica at Saint-Vaast-la-Hougue and Utah Beach, respectively, and 90 and 75 for nitrogen. These distributions are consistent with the classification shown in Fig. 13, which groups observations into six categories based on the relative order of nutrient limitation. Notably, the nutrient limitation patterns at Saint-Vaast-la-Hougue and Utah Beach are highly similar, with only slight differences in the relative frequencies of the P < Si < N and N < Si < P categories.
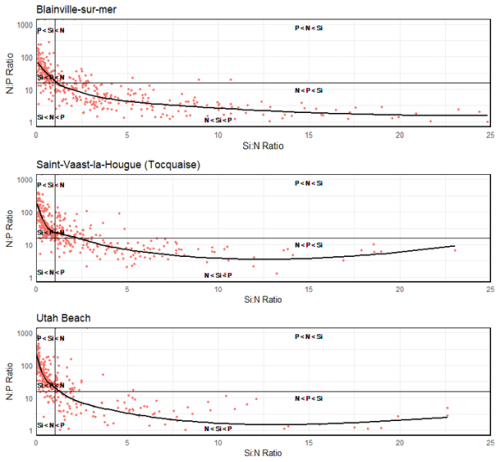
Figure 13Synthetic graph showing the molar ratios of Si : N : P from 2000 to 2024 at Blainville-sur-Mer, Saint-Vaast-la-Hougue, and Utah Beach. The plot is divided into six regions based on stoichiometric thresholds for biogenic particles, following Redfield et al. (1963) and Brzezinski (1985) (Si : N : P = 16 : 16 : 1). These regions indicate the order of priority for potentially limiting nutrients in each ecosystem.
The results of this study highlight the importance of conducting long-term monitoring at specific sites to better understand intra- and inter-annual variations in the biogeochemical cycles of nutrients and the evolution of physico-chemical parameters.
4.1 Data quality and limitations
The dataset compiled for this study is based on consistent sampling and analytical protocols applied from 2000 to 2024 across three coastal stations in Normandy. Sampling was conducted bi-monthly under standardized conditions, and nutrient analyses followed validated spectrophotometric methods in accordance with French and international standards (e.g., AFNOR XP T90-210). Quality assurance procedures included daily calibration of instruments, use of certified reference materials, and strict control over sample storage and processing times to limit degradation. Detection and quantification limits were determined for each parameter and are provided in Table 2 to support the interpretation of low-concentration values.
Despite the methodological rigor, some limitations should be acknowledged. Occasional data gaps are present, primarily due to adverse weather conditions or logistical constraints. Moreover, while the protocols ensure a high degree of comparability over time, analytical sensitivity may have evolved slightly over the 24-year period due to changes in equipment and detection capabilities. Finally, although extreme outliers were rare and handled using robust statistical methods, a small number of values were excluded following established quality control criteria. Overall, the dataset is considered highly reliable for assessing long-term stoichiometric trends and nutrient limitation patterns in the studied ecosystems
4.2 Coastal ecosystems and climate change
The three studied sites, located in the Normandy region, show a progressive increase in winter temperatures over the past decade (Figs. 2 and 3). This finding aligns with observations made at other monitoring points in the English Channel (Cornes et al., 2023; Kassem et al., 2023; McEvoy et al., 2023; Hubert et al., 2025; Neven et al., 2024). While our data indicate an average rise of 1 °C over 12 years for the three stations, studies conducted along the southern coast of England report an increase ranging from 0.42 to 0.76 °C per decade (Kassem et al., 2023). In France, Hubert et al. (2025) reported a similar warming trend, estimated at +1.063 °C over 11 years. Moreover, the year 2022 was marked by an exceptional heatwave, recorded by Simon et al. (2023), with particularly high summer temperatures (Guinaldo et al., 2023; Hubert et al., 2025). This phenomenon was also observed within our monitoring network. However, natural temperature oscillations, such as the Atlantic Multidecadal Oscillation (AMO; Kerr, 2000), also affect conditions in the English Channel (Edwards et al., 2013; Auber et al., 2017).
The warming of coastal waters may lead to significant changes in marine ecosystems, particularly by altering the composition and biomass of phytoplankton communities (Richardson and Schoeman, 2004), zooplankton (Neven et al., 2024), and fish populations (Auber et al., 2017; Maltby et al., 2020). These changes could have major repercussions on the dynamics of coastal ecosystems and the services they provide. Another major factor threatening marine ecosystems is ocean acidification. Observations and modeling studies indicate a global decrease in ocean pH by 0.02 units per decade, with a projected drop of up to −0.7 units by 2100 due to the dissolution of atmospheric CO2 into the oceans (Caldeira and Wickett, 2003; Bates, 2007; Santana-Casiano et al., 2007; Ólafsson et al., 2009; Lauvset et al., 2016). Such acidification will have significant consequences, including altering the structure of marine communities (Fabry et al., 2008), disrupting nutrient cycles (Hutchins et al., 2009), reducing productivity (Riebesell et al., 2007), and impacting carbon fluxes (Schulz et al., 2008).
Data from the HYDRONOR observatory reveal an average acidification over 24 years of −0.2 units in Blainville-sur-Mer, −0.25 units in Saint-Vaast-la-Hougue, and −0.15 units in Utah Beach. These values significantly exceed previously predicted levels, highlighting the urgency of better understanding these phenomena. Long-term monitoring of coastal ecosystems is crucial to understanding the effects of climate change, particularly in shellfish farming areas where bivalves, vulnerable due to their calcareous shells, are heavily impacted by acidification (Doney et al., 2020). However, coastal zones present specific challenges due to their high daily, seasonal, and interannual variability. This complexity, influenced by factors such as riverine inputs, climatic conditions, and anthropogenic pressures, makes identifying climate change-related trends more difficult (Kapsenberg et al., 2017; Reimer et al., 2017; Chen and Hu, 2019).
4.3 Ecological contrasts between the west and east coasts of Cotentin
Our findings highlight two distinct ecological dynamics between the West and East coasts of Cotentin. The West coast, represented by the Blainville-sur-Mer station, is characterized by an open environment where processes appear to “dilute”, leading to consistent and regular seasonal trends. As shown in Fig. 4, chlorophyll a peaks occur systematically in March, with variations mainly limited to the amplitude of phytoplankton blooms.
In contrast, the East coast exhibits much less predictable and highly variable trends from year to year. Regarding dissolved nutrients, although maximum concentrations are similar between the two coasts (except for ammonium, Fig. 6), these peaks are short-lived on the West coast.
We hypothesize that this “dilution” of processes on the West coast is linked to its open environment, with greater exchange with the English Channel and the Atlantic Ocean. Conversely, the East coast, influenced by the proximity of bays and riverine inputs (Fig. 1), experiences more intense and irregular phenomena. A notable difference lies in the limiting elements: the West coast is primarily nitrogen-deficient, whereas the East coast is limited by phosphorus and silica (Fig. 13). These observations contrast with studies conducted in the English Channel, which generally report a nitrogen surplus due to agricultural activities and riverine inputs (Ménesguen et al., 2019; Romero et al., 2019; Yan et al., 2021, 2022).
The PCA results (Figs. 10–12) support and further illustrate these contrasting ecological dynamics. At Blainville-sur-Mer (West coast, Fig. 10), the seasonal structure appears particularly regular and well-defined. The PCA shows a clear separation of winter and spring samples along the first principal component (Dim1), primarily driven by nutrient concentrations and their inverse relationship with temperature, salinity, and biological activity indicators such as chlorophyll a and dissolved oxygen. This regular structure aligns with the hypothesis of a diluted, open system dominated by predictable seasonal patterns and nutrient cycling. In contrast, at Saint-Vaast-la-Hougue and Utah Beach (East coast, Figs. 11 and 12), the PCA reveals greater seasonal dispersion and overlap, especially in spring and summer, indicating more variable and less predictable environmental conditions. These stations show a more complex multivariate structure, with spring samples, in particular, spreading across both axes, reflecting the influence of both nutrient enrichment and variable biological responses. The position of ammonium, weakly correlated with Dim1 at both East coast stations, suggests localized, irregular inputs, possibly linked to anthropogenic or benthic sources. Moreover, the nutrient vectors in the correlation circles are generally longer and more dominant in the East coast stations, especially at Saint-Vaast, suggesting that nutrient variability plays a stronger role in structuring the ecosystem in these areas. This supports the idea of more intense, irregular phenomena on the East coast, likely influenced by confined hydrodynamics, river discharges, and bay retention.
Differences between the ecosystems of the East and West coasts of the Cotentin Peninsula have already been highlighted in previous studies. Lefebvre et al. (2009a, b) found that the diet of oysters on the West coast was primarily based on phytoplankton (i.e., pelagic), whereas on the East coast, the animals consumed a mix of benthic and pelagic sources.
4.4 Drivers of long-term nutrient decline in coastal waters
Since the 1980s, European policies have aimed to reduce nutrient inputs, particularly phosphates and nitrates, from agricultural sources (Claussen et al., 2009; Garnier et al., 2019). Long-term analyses confirm that phytoplankton biomass is closely linked to both nutrient availability and hydroclimatic conditions (Loebl et al., 2009). Over the study period, a general decrease in dissolved nutrient concentrations – including ammonium, nitrate, nitrite, phosphate, and silicate – was observed across all three stations (Figs. 8 and 9). This reduction coincides with a widespread decline in chlorophyll a concentrations since the early 2000s (Goberville et al., 2010; Gohin et al., 2019), as illustrated in Fig. 7.
The decline in nitrogen and phosphorus concentrations is consistent with the implementation of agricultural regulations, including fertilizer use restrictions and wastewater treatment improvements (Claussen et al., 2009; Garnier et al., 2019). However, silicate is not directly influenced by such human inputs, as it mainly originates from the chemical weathering of continental rocks and is transported to the coastal zone via river discharge (Nelson and Gordon, 1982; Tréguer and De La Rocha, 2013).
The observed decrease in silicate concentrations therefore suggests a reduction in freshwater inflows to the coastal zone, potentially linked to lower river discharge. While hydrological data are sparse at the local scale, national assessments by ONEMA (Giuntoli et al., 2012) indicate that no significant trends in annual mean flows were detected in northern France between 1968 and 2007, in contrast to marked decreases in southern regions. However, recent climate trends – including increased evapotranspiration, altered precipitation patterns, and higher interannual variability - could still result in seasonal or event-scale reductions in riverine inputs to the coastal zone, which may not be captured by annual averages (Collet et al., 2014; Mimeau et al., 2025).
Additionally, anthropogenic alterations to watersheds, such as damming and land-use changes, have been shown to impact the silicon cycle by reducing dissolved silica export to estuaries (Ittekkot et al., 2006; Laruelle et al., 2009; Bernard et al., 2011). These changes, combined with climate-driven alterations in hydrology (Bernard et al., 2010), suggest that the silicon cycle in coastal ecosystems is highly sensitive to both direct human modification and indirect climate forcing, potentially affecting diatom-based primary production and broader food web dynamics.
The hydrobiological dataset from the HYDRONOR Observatory is openly accessible on Zenodo at the following DOI: https://doi.org/10.5281/zenodo.15058835 (SMEL, 2024). Additionally, the R package TTAinterfaceTrendAnalysis, designed for trend analysis, is available for consultation and download directly from the CRAN website (Devreker and Lefebvre, 2014; R Core Team, 2022; https://cran.r-project.org/web/packages/TTAinterfaceTrendAnalysis/index.html).
This study provides valuable insights into the long-term trends of hydrobiological parameters in Normandy's shellfish ecosystems, emphasizing the significant impacts of both human activity and climate change. Our analysis highlights a consistent increase in winter temperatures and a gradual acidification of pH levels across all three monitoring stations (Blainville-sur-Mer, Saint-Vaast-la-Hougue, and Utah Beach) from 2000 to 2024. These changes align with broader trends observed in the English Channel and suggest a shift in the environmental conditions that could affect the ecological balance of coastal habitats.
The observed variations in chlorophyll a concentrations and nutrient dynamics reveal complex interactions between nutrient availability, primary production, and the potential limitations of different nutrients across seasons. Notably, nitrogen was identified as the primary limiting nutrient in the western region, while phosphorus dominated as the limiting factor in the eastern stations. These findings have important implications for managing nutrient inputs and maintaining the health of coastal ecosystems, particularly in the context of ongoing eutrophication.
Furthermore, the study underscores the importance of sustained, site-specific monitoring to capture the intricate and evolving dynamics of coastal environments. The long-term dataset from the HYDRONOR observatory has proven essential in understanding how climatic shifts, such as increased temperatures, influence phytoplankton blooms and nutrient cycling. This research provides a crucial foundation for future efforts to mitigate the impacts of climate change and anthropogenic pressures on coastal ecosystems, particularly those supporting critical industries like shellfish farming. As the region faces rising temperatures and ongoing nutrient imbalances, adaptive management strategies will be key to preserving the resilience of these vital ecosystems.
JS wrote the paper. JB and SP coordinate the ecological monitoring of the HYDRONOR observatory. SP developed all the methodologies and has conducted all laboratory analyses since 2000.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Also, please note that this paper has not received English language copy-editing. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We express our gratitude to all the SMEL agents who have contributed to hydrobiological monitoring over the years through their dedicated efforts in data collection and fieldwork.
This study was supported by the HYDRONOR observatory, which is funded by SMEL's own resources (Departmental Council of Manche, CD50).
This paper was edited by Sabine Schmidt and reviewed by Sébastien Lefebvre and one anonymous referee.
Aminot, A. and Chaussepied, M.: Manuel des analyses chimiques en milieu marin, Ifremer, ISBN 978-2-902721-10-8, 1983.
Aminot, A. and Kérouel, R.: Hydrologie des écosystèmes marins: paramètres et analyses, Éditions Quae, ISBN 978-2-84433-133-5, 2004.
Auber, A., Gohin, F., Goascoz, N., and Schlaich, I.: Decline of cold-water fish species in the Bay of Somme (English Channel, France) in response to ocean warming, Estuar. Coast. Shelf Sci., 189, 189–202, https://doi.org/10.1016/j.ecss.2017.03.004, 2017.
Barbier, E. B., Hacker, S. D., Kennedy, C., Koch, E. W., Stier, A. C., and Silliman, B. R.: The value of estuarine and coastal ecosystem services, Ecol. Monogr., 81, 169–193, https://doi.org/10.1890/10-1510.1, 2011.
Bates, N. R.: Interannual variability of the oceanic CO2 sink in the subtropical gyre of the North Atlantic Ocean over the last 2 decades, J. Geophys. Res.-Oceans, 112, C09013, https://doi.org/10.1029/2006JC003759, 2007.
Beaugrand, G.: Monitoring marine plankton ecosystems. I: Description of an ecosystem approach based on plankton indicators, Mar. Ecol.-Prog. Ser., 269, 69–81, https://doi.org/10.3354/meps269069, 2004.
Bendschneider, K. and Robinson, R. J.: A new spectrophotometric method for the determination of nitrite in sea water, J. Mar. Res., 11, 87–96, 1952.
Bernard, C. Y., Laruelle, G. G., Slomp, C. P., and Heinze, C.: Impact of changes in river fluxes of silica on the global marine silicon cycle: a model comparison, Biogeosciences, 7, 441–453, https://doi.org/10.5194/bg-7-441-2010, 2010.
Bernard, C. Y., Durr, H. H., Heinze, C., Segschneider, J., and Meier-Reimer, E.: Contribution of riverine nutrients to the silicon biogeochemistry in the global ocean – a model study, Biogeosciences, 8, 551–564, https://doi.org/10.5194/bg-8-551-2011, 2011.
Brzezinski, M. A.: The Si : C : N ratio of marine diatoms: interspecific variability and the effect of some environmental variables, J. Phycol., 21, 347–357, https://doi.org/10.1111/j.0022-3646.1985.00347.x, 1985.
Cadée, G. C. and Hegeman, J.: Phytoplankton in the Marsdiep at the end of the 20th century: 30 years monitoring biomass, primary production, and Phaeocystis blooms, J. Sea Res., 48, 97–110, https://doi.org/10.1016/S1385-1101(02)00161-2, 2002.
Caldeira, K. and Wickett, M. E.: Anthropogenic carbon and ocean pH, Nature, 425, 365, https://doi.org/10.1038/425365a, 2003.
Chen, S. and Hu, C.: Environmental controls of surface water pCO2 in different coastal environments: observations from marine buoys, Cont. Shelf Res., 183, 73–86, https://doi.org/10.1016/j.csr.2019.05.007, 2019.
Chenel, J.: Étude du compartiment “chimie” du projet CarUtah, MS thesis, SMEL – Synergie Mer et Littoral, https://www.smel.fr/download/etude-du-compartiment-chimie-du-projet-carutah-stage-m2-julie-chenel/ (last access: 16 September 2025), 2025.
Claussen, U., Zevenboom, W., Brockmann, U., Topcu, D., and Bot, P.: Assessment of the eutrophication status of transitional, coastal and marine waters within OSPAR, Hydrobiologia, 629, 49–58, https://doi.org/10.1007/s10750-009-9763-3, 2009.
Cloern, J. E.: Our evolving conceptual model of the coastal eutrophication problem, Mar. Ecol.-Prog. Ser., 210, 223–253, https://doi.org/10.3354/meps210223, 2001.
Collet, L., Ruelland, D., Borrell-Estupina, V., and Servat, E.: Variabilité climatique et des activités humaines sur les ressources en eau d'un bassin Méditerranéen de méso-échelle, Hydrolog. Sci. J., 59, 1457–1469, https://doi.org/10.1080/02626667.2013.842073, 2014.
Cornes, R. C., Tinker, J., Hermanson, L., Oltmanns, M., Hunter, W. R., Lloyd-Hartley, H., Kent, E. C., Rabe, B., and Renshaw, R.: The impacts of climate change on sea temperature around the UK and Ireland, Mar. Climate Change Impacts Partnership, 18 pp., https://nora.nerc.ac.uk/id/eprint/534103/ (last access: 16 September 2025), 2023.
Devreker, D. and Lefebvre, A.: TTAinterfaceTrendAnalysis: a R GUI for routine temporal trend analysis and diagnostics, J. Oceanogr. Res. Data, 6, 1–18, 2014 (code available at: https://cran.r-project.org/web/packages/TTAinterfaceTrendAnalysis/index.html, last access: 16 September 2025).
Diaz, R. J.: Overview of hypoxia around the world, J. Environ. Qual., 30, 275–281, https://doi.org/10.2134/jeq2001.302275x, 2001.
Doney, S. C., Busch, D. S., Cooley, S. R., and Kroeker, K. J.: The impacts of ocean acidification on marine ecosystems and reliant human communities, Annu. Rev. Environ. Resour., 45, 83–112, https://doi.org/10.1146/annurev-environ-012320-083019, 2020.
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z. I., Knowler, D. J., Lévêque, C., Naiman, R. J., Prieur-Richard, A. H., Soto, D., Stiassny, M. L. J., and Sullivan, C. A. Freshwater biodiversity: importance, threats, status and conservation challenges, Biol. Rev., 81, 163–182, https://doi.org/10.1017/S1464793105006950, 2006.
Edwards, M., Beaugrand, G., Helaouët, P., Alheit, J., and Coombs, S.: Marine ecosystem response to the Atlantic Multidecadal Oscillation, PLOS ONE, 8, e57212, https://doi.org/10.1371/journal.pone.0057212, 2013.
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C.: Impacts of ocean acidification on marine fauna and ecosystem processes, ICES J. Mar. Sci., 65, 414–432, https://doi.org/10.1093/icesjms/fsn048, 2008.
Filgueira, R., Guyondet, T., Comeau, L. A., and Tremblay, R.: Bivalve aquaculture–environment interactions in the context of climate change, Global Change Biol., 22, 3901–3913, https://doi.org/10.1111/gcb.13346, 2016.
Garnier, J., Riou, P., Le Gendre, R., Ramarson, A., Billen, G., Cugier, P., Schapira, M., Thery, S., Thieu, V., and Menesguen, A.: Managing the agri-food system of watersheds to combat coastal eutrophication: a land-to-sea modelling approach to the French Coastal English Channel, Geosciences, 9, 441, https://doi.org/10.3390/geosciences9100441, 2019.
Giuntoli, I., Maugis, P., and Renard, B.: Évolutions observées dans les débits des rivières en France: sélection d'un réseau de référence et analyse de l'évolution temporelle des régimes des 40 dernières années, Collection “Comprendre pour agir”, Onema, p. 8, https://hal.science/hal-02936887v1 (last access: 16 September 2025), 2012.
Goberville, E., Beaugrand, G., Sautour, B., Tréguer, P., and Somelit team: Climate-driven changes in coastal marine systems of western Europe, Mar. Ecol.-Prog. Ser., 408, 129–147, https://doi.org/10.3354/meps08564, 2010.
Gohin, F., Van der Zande, D., Tilstone, G., Eleveld, M. A., Lefebvre, A., Andrieux-Loyer, F., Blauw, A. N., Bryère, P., Devreker, D., Garnesson, P., Hernandez Farinas, T., Lamaury, Y., Lampert, L., Lavigne, H., Menet-Nedelec, F., Pardo, S., and Saulquin, B.: Twenty years of satellite and in situ observations of surface chlorophyll-a from the northern Bay of Biscay to the eastern English Channel: is the water quality improving?, Remote Sens. Environ., 233, 111343, https://doi.org/10.1016/j.rse.2019.111343, 2019.
Grangere, K.: Simulation de l'influence des apports des bassins versants sur les concessions ostréicoles de la Baie des Veys (Baie de Seine Occidentale), Post-graduate thesis, DEA) University of Caen, https://archimer.ifremer.fr/doc/00449/56100/ (last access: 16 September 2025), 2004.
Guinaldo, T., Voldoire, A., Waldman, R., Saux Picart, S., and Roquet, H.: Response of the sea surface temperature to heatwaves during the France 2022 meteorological summer, Ocean Sci., 19, 629–647, https://doi.org/10.5194/os-19-629-2023, 2023.
Halpern, B. S., Selkoe, K. A., Micheli, F., and Kappel, C. V.: Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats, Conserv. Biol., 21, 1301–1315, https://doi.org/10.1111/j.1523-1739.2007.00752.x, 2007.
Heisler, J., Glibert, P. M., Burkholder, J. M., Anderson, D. M., Cochlan, W., Dennison, W. C., Dortch, Q., Gobler, C. J., Heil, C. A., Humphries, E., Lewitus, A., Magnien, R., Marshall, H. G., Sellner, K., Stockwell, D. A., Stoecker, D. K., and Suddleson, M.: Eutrophication and harmful algal blooms: a scientific consensus, Harmful Algae, 8, 3–13, https://doi.org/10.1016/j.hal.2008.08.006, 2008.
Hubert, Z., Louchart, A. P., Robache, K., Epinoux, A., Gallot, C., Cornille, V., Crouvoisier, M., Monchy, S., and Artigas, L. F.: Decadal changes in phytoplankton functional composition in the Eastern English Channel: possible upcoming major effects of climate change, Ocean Sci., 21, 679–700, https://doi.org/10.5194/os-21-679-2025, 2025.
Hutchins, D. A., Mulholland, M. R., and Fu, F.: Nutrient cycles and marine microbes in a CO2-enriched ocean, Oceanography, 22, 128–145, https://doi.org/10.5670/oceanog.2009.103, 2009.
Ittekkot, V., Unger, D., Humborg, C., and An, N. T. (Eds.): The silicon cycle: human perturbations and impacts on aquatic systems, in: SCOPE Series Vol. 66, Island Press, 296 pp., ISBN 978-1597261159, 2006.
Kapsenberg, L., Alliouane, S., Gazeau, F., Mousseau, L., and Gattuso, J.-P.: Coastal ocean acidification and increasing total alkalinity in the northwestern Mediterranean Sea, Ocean Sci., 13, 411–426, https://doi.org/10.5194/os-13-411-2017, 2017.
Kassem, H., Amos, C. L., and Thompson, C. E. L.: Sea surface temperature trends in the coastal zone of southern England, J. Coast. Res., 39, 18–31, https://doi.org/10.2112/JCOASTRES-D-22-00056.1, 2023.
Kerr, R. A.: A North Atlantic climate pacemaker for the centuries, Science, 288, 1984–1985, https://doi.org/10.1126/science.288.5473.1984, 2000.
Kirby, R., Beaugrand, G., and Lindley, J. A.: Synergistic effects of climate and fishing in a marine ecosystem, Ecosystems, 12, 548–561, https://doi.org/10.1007/s10021-009-9241-9, 2009.
Laruelle, G. G., Roubeix, V., Sferratore, A., Brodherr, B., and Ciuffa, D.: Anthropogenic perturbations of the silicon cycle at the global scale: Key role of the land-ocean transition, Global Biogeochem. Cy., 23, GB4031, https://doi.org/10.1029/2008GB003267, 2009.
Lauvset, S. K., Key, R. M., Olsen, A., van Heuven, S., Velo, A., Lin, X., Schirnick, C., Kozyr, A., Tanhua, T., Hoppema, M., Jutterström, S., Steinfeldt, R., Jeansson, E., Ishii, M., Pérez, F. F., Suzuki, T., and Watelet, S.: A new global interior ocean mapped climatology: The 1×1 GLODAP version 2, Earth Syst. Sci. Data, 8, 325–340, https://doi.org/10.5194/essd-8-325-2016, 2016.
Lefebvre, S., Marín Leal, J. C., Dubois, S., Orvain, F., Blin, J. L., Bataillé, M. P., Ourry, A., and Galois, R.: Seasonal dynamics of trophic relationships among co-occurring suspension feeders in two shellfish culture dominated ecosystems, Estuar. Coast. Shelf Sci., 82, 415–425, https://doi.org/10.1016/j.ecss.2009.02.026, 2009a.
Lefebvre, S., Harma, C., and Blin, J. L.: Trophic typology of coastal ecosystems based on δ13C and δ15N ratios in an opportunistic suspension feeder, Mar. Ecol.-Prog. Ser., 390, 27–37, https://doi.org/10.3354/meps08170, 2009b.
Lefebvre, A., Guiselin, N., Barbet, F., and Artigas, L. F.: Long-term hydrological and phytoplankton monitoring (1992–2007) of three potentially eutrophicated systems in the eastern English Channel and the southern bight of the North Sea, ICES J. Mar. Sci., 68, 2029–2043, https://doi.org/10.1093/icesjms/fsr151, 2011.
Leruste, A., Pasqualini, V., Garrido, M., Malet, N., De Wit, R., and Bec, B.: Physiological and behavioral responses of phytoplankton communities to nutrient availability in a disturbed Mediterranean coastal lagoon, Estuar. Coast. Shelf Sci., 219, 176–188, https://doi.org/10.1016/j.ecss.2019.01.009, 2019.
Loebl, M., Colijn, F., van Beusekom, J. E. E., Baretta-Bekker, J. G., Lancelot, C., Philippart, C. J. M., Rousseau, V., and Wiltshire, K. H.: Recent patterns in potential limitation along the Northwest European continental coast, J. Sea Res., 61, 34–43, https://doi.org/10.1016/j.seares.2008.05.002, 2009.
Maltby, K. M., Rutterford, L. A., Tinker, J., Genner, M. J., and Simpson, S. D.: Projected impacts of warming seas on commercially fished species at a biogeographic boundary of the European continental shelf, J. Appl. Ecol., 57, 2222–2233, doi10.1111/1365-2664.13682, 2020.
Martin, G. D., Vijay, J. G., Laluraj, C. M., Madhu, N. V., Joseph, T., Nair, M., Gupta, G. V. M., and Balachandran, K. K.: Fresh water influence on nutrient stoichiometry in a tropical estuary, southwest coast of India, Appl. Ecol. Environ. Res., 6, 57–64, https://doi.org/10.15666/aeer/0601_057064, 2008.
McEvoy, A. J., Atkinson, A., Airs, R. L., Brittain, R., Brown, I., Fileman, E. S., Findlay, H. S., McNeil, C. L., Ostle, C., Smyth, T. K., Somerfield, P. J., Tait, K., Tarran, G. A., Thomas, S., Widdicombe, C. E., Woodward, E., Beesley, A., Conway, D. V. P., Fishwick, J., and Widdicombe, S.: The Western Channel Observatory: A century of physical, chemical and biological data compiled from pelagic and benthic habitats in the western English Channel, Earth Syst. Sci. Data, 15, 5701–5737, https://doi.org/10.5194/essd-15-5701-2023, 2023.
Ménesguen, A., Dussauze, M., Dumas, F., Thouvenin, B., Garnier, V., Lecornu, F., and Répécaud, M.: Ecological model of the Bay of Biscay and English Channel shelf for environmental status assessment. Part 1: Nutrients, phytoplankton and oxygen, Ocean Model., 133, 56–78, https://doi.org/10.1016/j.ocemod.2018.11.005, 2019.
Meybeck, M., Lestel, L., Carré, C., Bouleau, G., Garnier, J., and Mouchel, J. M.: Trajectories of river chemical quality issues over the longue durée: The Seine River (1900s–2010), Environ. Sci. Pollut. R., 25, 23468–23484, https://doi.org/10.1007/s11356-016-7124-0, 2018.
Mimeau, L., Künne, A., Devers, A., Branger, F., Kralisch, S., Lauvernet, C., Vidal, J.-P., Bonada, N., Csabai, Z., Mykrä, H., Pařil, P., Polović, L., and Datry, T.: Projections of streamflow intermittence under climate change in European drying river networks, Hydrol. Earth Syst. Sci., 29, 1615–1636, https://doi.org/10.5194/hess-29-1615-2025, 2025.
Mullin, J. and Riley, J. P.: The colorimetric determination of silicate with special reference to sea and natural waters, Anal. Chim. Ac., 12, 162–176, https://doi.org/10.1016/S0003-2670(00)87825-3, 1955.
Murphy, J. and Riley, J. P.: A modified single solution method for the determination of phosphate in natural waters, Anal. Chim. Ac., 27, 31–36, https://doi.org/10.1016/S0003-2670(00)88444-5, 1962.
Nelson, D. M. and Gordon, L. I.: Production and pelagic dissolution of biogenic silica in the Southern Ocean, Geochim. Cosmochim. Ac., 46, 491–501, https://doi.org/10.1016/0016-7037(82)90153-3, 1982.
Neven, C. J., Giraldo, C., Girardin, R., Lefebvre, A., Lefebvre, S., Loots, C., Meunier, C. L., and Marchal, P.: Winter distribution of zooplankton and ichthyoplankton assemblages in the North Sea and the English Channel, PLoS ONE, 19, e0308803, https://doi.org/10.1371/journal.pone.0308803, 2024.
Ólafsson, J., Ólafsdóttir, S. R., Benoit-Cattin, A., Danielsen, M., Arnarson, T. S., and Takahashi, T.: Rate of Iceland Sea acidification from time series measurements, Biogeosciences, 6, 2661–2668, https://doi.org/10.5194/bg-6-2661-2009, 2009.
Ovaskainen, O., Weigel, B., Potyutko, O., and Buyvolov, Y.: Long-term shifts in water quality show scale-dependent bioindicator responses across Russia – Insights from 40 year-long bioindicator monitoring program, Ecol. Indic., 98, 476–482, https://doi.org/10.1016/j.ecolind.2018.11.027, 2019.
R Core Team: R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (last access: 16 September 2025), 2022.
Redfield, A. C., Ketchum, B. H., and Richards, F. A.: The influence of organisms on composition of seawater, in: The Sea, vol. 2, edited by: Hill, M. N., Interscience, New York, 26–77, ISBN 978-0674017283, 1963.
Reid, P. C., Edwards, M., Beaugrand, G., Skogen, M., and Stevens, D.: Periodic changes in the zooplankton of the North Sea during the twentieth century linked to oceanic inflow, Fish. Oceanogr., 12, 260–269, https://doi.org/10.1046/j.1365-2419.2003.00252.x, 2003.
Reimer, J. J., Cai, W.-J., Xue, L., Vargas, R., Noakes, S., Hu, X., Signorini, S. R., Mathis, J. T., Feely, R. A., Sutton, A. J., Sabine, C., Musielewicz, S., Chen, B., and Wanninkhof, R.: Time series pCO2 at a coastal mooring: Internal consistency, seasonal cycles, and interannual variability, Cont. Shelf Res., 145, 95–108, https://doi.org/10.1016/j.csr.2017.06.022, 2017.
Richardson, A. J. and Schoeman, D. S.: Climate impact on plankton ecosystems in the Northeast Atlantic, Science, 305, 1609–1612, https://doi.org/10.1126/science.1100958, 2004.
Riebesell, U., Schulz, K. G., Bellerby, R. G. J., Botros, M., Fritsche, P., Meyerhöfer, M., Neil, C., Nondal, G., Wohlers, J., and Zöllner, E.: Enhanced biological carbon consumption in a high CO2 ocean, Nature, 450, 545–548, https://doi.org/10.1038/nature06267, 2007.
Romero, E., Le Gendre, R., Garnier, J., Billen, G., Fisson, C., Silvestre, M., and Riou, P.: Long-term water quality in the lower Seine: Lessons learned over 4 decades of monitoring, Environ. Sci. Policy, 141–154, https://doi.org/10.1016/j.envsci.2016.01.016, 2016.
Romero, E., Garnier, J., Billen, G., Ramarson, A., Riou, P., and Le Gendre, R.: Modeling the biogeochemical functioning of the Seine estuary and its coastal zone: Export, retention, and transformations, Limnol. Oceanogr., 64, 895–912, https://doi.org/10.1002/lno.11082, 2019.
Sala, O. E., Chapin, F. S., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L. F., Jackson, R. B., Kinzig, A., Leemans, R., Lodge, D. M., Mooney, H. A., Oesterheld, M., Poff, N. L. R., Sykes, M. T., Walker, B. H., Walker, M., and Wall, D. H.: Global biodiversity scenarios for the year 2100, Science, 287, 1770–1774, https://doi.org/10.1126/science.287.5459.1770, 2000.
Santana-Casiano, J. M., González-Dávila, M., Rueda, M. J., Llinás, O., and González-Dávila, E. F.: The interannual variability of oceanic CO2 parameters in the northeast Atlantic subtropical gyre at the ESTOC site, Global Biogeochem. Cy., 21, GB1015, https://doi.org/10.1029/2006GB002788, 2007.
Sarmiento, J. L. and Gruber, N.: Ocean biogeochemical dynamics, Princeton University Press, ISBN 978-0691017075, 2006.
Schulz, K. G., Riebesell, U., Bellerby, R. G. J., Biswas, H., Meyerhöfer, M., Müller, M. N., Egge, J. K., Nejstgaard, J. C., Neil, C., Wohlers, J., and Zöllner, E.: Build-up and decline of organic matter during PeECE III, Biogeosciences, 5, 707–718, https://doi.org/10.5194/bg-5-707-2008, 2008.
Selman, M., Greenhalgh, S., Diaz, R., and Sugg, Z.: Eutrophication and hypoxia in coastal areas: A global assessment of the state of knowledge, World Resources Institute, ISBN 978-1-56973-681-4, 2008.
Shen, Z. L.: Historical changes in nutrient structure and its influences on phytoplankton composition in Jiaozhou Bay, Estuar. Coast. Shelf Sci., 52, 211–224, https://doi.org/10.1006/ecss.2000.0736, 2001.
Simon, A., Poppeschi, C., Plecha, S., Charria, G., and Russo, A.: Coastal and regional marine heatwaves and cold spells in the northeastern Atlantic, Ocean Sci., 19, 1339–1355, https://doi.org/10.5194/os-19-1339-2023, 2023.
SMEL – Synergie Mer and Littoral: Hydrobiological data from 6 stations of the HYDRONOR Observatory (2000–2024), Zenodo [data set], https://doi.org/10.5281/zenodo.15058835, 2024.
Smith, V. H.: Responses of estuarine and coastal marine phytoplankton to nitrogen and phosphorus enrichment, Limnol. Oceanogr., 51, 377–384, https://doi.org/10.4319/lo.2006.51.1_part_2.0377, 2006.
Sonier, R., Filgueira, R., Guyondet, T., Tremblay, R., Olivier, F., Meziane, T., Starr, M., LeBlanc, A. R., and Comeau, L. A.: Picophytoplankton contribution to Mytilus edulis growth in an intensive culture environment, Mar. Biol., 163, 1–15, 2016.
Strickland, J. and Parsons, T.: A practical handbook of seawater analysis, in: 2nd Edn., Vol. 167, Fisheries Research Board of Canada, ISBN 978-0660115962, 1972.
Tréguer, P. J. and De La Rocha, C. L. The world ocean silica cycle, Annu. Rev. Mar. Sci., 5, 477–501, https://doi.org/10.1146/annurev-marine-121211-172346, 2013.
Vermaat, J. E., McQuatters-Gollop, A., Eleveld, M. A., and Gilbert, A.: Past, present and future nutrient loads of the North Sea: Causes and consequences, Estuar. Coast. Shelf Sci., 80, 53–59, 2008.
Watanabe, K., Kasai, A., Fukuzaki, K., Ueno, M., and Yamashita, Y.: Estuarine circulation-driven entrainment of oceanic nutrients fuels coastal phytoplankton in an open coastal system in Japan, Estuar. Coast. Shelf Sci., 184, 126–137, https://doi.org/10.1016/j.ecss.2016.10.031, 2017.
Yan, X., Thieu, V., and Garnier, J.: Long-term assessment of nutrient budgets for the four reservoirs of the Seine Basin (France), Sci. Total Environ., 778, 1, https://doi.org/10.1016/j.scitotenv.2021.146412, 2021.
Yan, X., Garnier, J., Billen, G., Wang, S., and Thieu, V.: Unravelling nutrient fate and CO2 concentrations in the reservoirs of the Seine Basin using a modelling approach, Water Res., 225, 119135, https://doi.org/10.1016/j.watres.2022.119135, 2022.